|
|
|
Indian Pediatr 2015;52:
957-960 |
 |
Neurodevelopmental Status of Children Aged
6-30 Months With Congenital Heart Disease
|
|
Kusum Lata, Devendra Mishra, *Vimal Mehta and Monica
Juneja
From Department of Pediatrics, Lok Nayak Hospital;
and *Department of Cardiology, GB Pant Hospital; Maulana Azad Medical
College, New Delhi, India.
Correspondence to: Dr Devendra Mishra, Departments of
Pediatrics, Maulana Azad Medical College, New Delhi, India. Email:
[email protected]
Received: December 26, 2014;
Initial review: January 27, 2015;
Accepted: August 28, 2015.
|
Background: Children with congenital heart diseases (CHD) are
considered to be at high-risk for neurodevelopmental delay, but scant
Indian data are available.
Objective: To evaluate the neurodevelopmental
status of children with CHD.
Methods: We enrolled consecutive children aged
6-30 months with echocardiographically-confirmed CHD between June 2013
and January 2014. Children with clinically recognizable genetic
syndromes or disorders; visual and/or hearing deficits, and microcephaly;
and post-cardiac surgery children were excluded. Development was
assessed by Developmental Assessment Scale for Indian Infants (DASII)
and Developmental delay defined as Development Quotient (DQ) <70 in
either the mental or motor scale.
Results: 75 children (53 males) with CHD were
enrolled. Acyanotic CHD was seen in 51 children (VSD in 47%), and
Tetralogy of Fallot was the commonest cyanotic CHD (25%). Developmental
delay was seen in 25% of these children, more in the motor domain (48%)
than in mental (12%). Mean motor and mental DQ in acyanotic CHD was 77
and 84, respectively; and 65 and 85, respectively in cyanotic CHD. Mean
motor DQ was significantly less than mental DQ in both acyanotic and
cyanotic CHD children (P=0.048).
Conclusion: Children with CHD are at an increased
risk for developmental delay. Periodic surveillance, screening and
evaluation should be instituted in them for early identification and
appropriate interventions to enhance later academic, behavioral,
psycho-social and adaptive function.
Keywords: Congenital heart defects, Developmental
disabilities, Intervention, Neurodevelopmental delay, Outcome,
Surveillance.
|
|
I
ncreasing survival rates in children with
Congenital heart diseases (CHD) have been associated with a shift in
focus from heart-related morbidity and death to concern for brain
integrity, and developmental and neurological outcomes have come under
increasing scrutiny [1-3]. These children are at risk of developmental
problems due to events that occur during intrauterine life, at surgery,
or during the growing years e.g., poor perfusion, shock,
acid-base disturbances, hypoxia, and failure to thrive.
Neurodevelopmental delay in children with CHD is reported to be more
common with cyanotic CHD, and in those requiring surgical intervention
[4].
There is very little information available on the
neurodevelopmental status of Indian children with CHD. The only Indian
study on the topic has addressed neurodevelopmental outcome of infants
after cardiac surgery [5]. Thus, the present study was conducted to
study the neurodevelopmental status of children with congenital heart
disease and elucidate associated factors.
Methods
This descriptive study was conducted from June 2013
to January 2014 in the Pediatrics department of Lok Nayak hospital,
Maulana Azad Medical College, Delhi, after obtaining clearance from the
Institutional Ethics Committee. With an expected 25% prevalence of
developmental delay in children with CHD, with a 90% precision and 95%
confidence, a sample size of 72 was calculated. We planned to enroll 75
children, expecting a 5% loss to follow-up between stabilization and
developmental assessment.
All consecutive children in the age group of 6 to 30
month who presented with symptoms and signs suggestive of congenital
heart disease, which was confirmed by echocardiography, were approached
for inclusion after initial management and stabilization of the child.
Children with clinically recognizable genetic syndromes or disorders
e.g., Down, Alagille, Turner or Noonan syndrome and VACTERL
association; Visual and/or hearing deficits; microcephaly; and, those
who were post-cardiac surgery were excluded. Parents were explained
about the purpose of the study and a written informed consent was
obtained. This process was continued till the a priori sample
size of 75 children was achieved.
Echocardiography was done after the patient was
stabilized, and details of the cardiac problem were recorded in the
form. Blood investigations including complete hemogram, serum calcium,
serum phosphorus, and alkaline phosphatase were done in all children.
Neurodevelopmental assessment was done by Developmental Assessment Scale
for Indian Infants (DASII) [6] by a single trained examiner, when the
child was clinically stable to undergo the evaluation. Developmental
delay was defined on DASII as DQ score
£70 (£2SD)
in either the mental or motor scale [6]. Anemia was defined as
hemoglobin <11g/100mL in acyanotic CHD group and <15g/100mL in cyanotic
CHD group. Management of the child’s acute condition was done by the
pediatricians in the treating unit. All children with developmental
delay also underwent thyroid function tests.
Clinical severity of lesion was classified as per
criteria suggested by Hoffman and Kaplan [7]. Children were classified
as low-, moderate- and high-risk groups for developmental delay based on
criteria given by American Heart Association [4]. Appropriate
inter-ventions were provided at the Child Development Centre of our
institution for all children with developmental delay; and early
intervention provided to those in high-risk categories for developmental
delay [8].
Statistical analysis: Data were entered in Excel
spreadsheets and analyzed using SPSS 16.0 by a statistician. Various
anthropometric and clinical factors were compared between CHD patients
with or without neurodevelopmental delay by the Chi-square test or
Fischer exact test. Mean DQ was compared between cyanotic and acyanotic
groups by the Student’s t test, and between risk and severity groups by
Anova. A P-value less than 0.05 was considered significant.
Results
A total of 2105 children in the described age-group
attended the hospital during the study period, of which 75 children (51
acyanotic CHD) were enrolled (Fig. 1). Majority of
children (62.6%) in the study group were in the younger age group (6-12
month), with 29.4% older than 18 months. 57.3% had weight <-3 Z score of
WHO charts; although there were no age, sex or anthropometric
differences between children with cyanotic or acyanotic CHD. Ventricular
septal defect was the commonest acyanotic CHD (47%) and Tetralogy of
Fallot was the commonest cyanotic CHD (25%) (Table I).
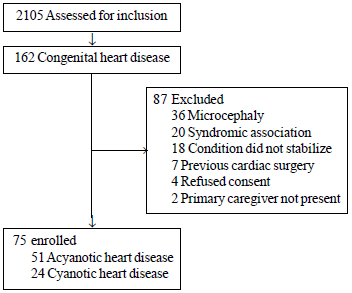 |
|
Fig.1 Flow of participants in the
study.
|
TABLE I Characteristics of Children With Congenital Heart Disease (N=75)
|
Characteristic |
ACHD (n=51), |
CCHD (n=24), |
|
No. (%) |
No. (%) |
|
Male sex |
34 (66.7) |
19 (79.2) |
|
Weight <-3 Z score |
34 (66.7) |
9 (37.5) |
|
Height <-3 Z score |
10 (19.6) |
2 (8.3) |
|
Anemia |
36 (70.6) |
17 (70.8) |
|
Hypocalcemia |
14 (27.4) |
6 (25.0) |
|
Rickets |
3 (5.8) |
3 (12.4) |
|
ACHD: Acyanotic, and CCHD: cyanotic congenital heart
disease. |
TABLE II Developmental Status in Children With Congenital Heart Disease (N=75)
|
Characteristic |
ACHD group (n=51 ) |
CCHD group (n=24 ) |
All children (n=75 ) |
|
Development Quotient, Mean (SD) |
|
Motor |
77 (17.9) |
65 (17.8) |
71 (17.9) |
|
Mental |
84 (11.8) |
85 (11.7) |
84.5 (11.8) |
|
Developmental Delay (DQ≤70), No.
(%) |
|
* Motor |
18 (35.3) |
18 (75) |
36 (48) |
|
Mental DQ |
8 (15.7) |
1 (4.2) |
9 (12) |
|
*P<0.001 for comparison of proportion of children with
cyanotic and acyanotic CHD with motor delay. |
TABLE III Developmental Status in Children With Congenital Heart Disease Based on Severity
Classification and Risk Stratification (N=75)#
|
Characteristic |
Motor DQ, |
Mental DQ, |
|
|
Mean (SD) |
Mean (SD) |
|
*Severity |
|
Mild (n=14) |
79 (17.9) |
85 (11.8) |
|
Moderate (n= 23) |
75 (17.8) |
88 (11.7) |
|
Severe (n=38 ) |
64 (17.8) |
86 (11.7) |
|
Risk |
|
Low (n=35) |
73 (17.9) |
87 (11.8) |
|
Moderate (n=31) |
70 (17.8) |
87 (11.7) |
|
High (n= 9) |
60 (17.8) |
83 (12.1) |
|
#Severity of congenital heart disease as per Hoffman
and Kaplan [7], and risk group as per American Heart Association
[4]; *For Motor DQ across severity groups P<0.01. |
The mean Motor DQ was significantly lower in the
cyanotic group than acyanotic group (P=0.048). However, mean
Mental DQ was not different among acyanotic and cyanotic CHD groups (P=0.92)
or according to type of acyanotic CHD (P = 0.44) (Table
II). Delayed motor development was seen in 75% children with
cyanotic CHD and 35.3% with acyanotic CHD (P=0.001) (Table
II). The motor DQ was also found to be significantly affected by
type of ACHD (P = 0.018), with lower motor DQ in large VSD and
complex acyanotic CHD. DQ was not different among various type of
cyanotic CHD (P=0.223 and 0.526 for mean Motor and Mental,
respectively) (Data not shown).
All cyanotic CHD children were in severe group (Table
III). As per AHA risk stratification for developmental delay,
35(68%) children were in low and 15 (29%) in moderate risk group among
acyanotic CHD. Among cyanotic CHD, 16 (66%) were in moderate risk group
and 8 (33%) in high risk group. As expected, motor DQ was found to be
significantly lower in the severe group (P=0.01), whereas mental
DQ was not much different across either the severity groups or the risk
categories (Table III).
Neurological abnormalities were found in 6 children,
among which 3 children were in high risk category. Most common
neurological abnormality found was hypotonia (5 children). USG cranium
was normal in all children.
Discussion
In this descriptive hospital-based study of 75
children with CHD (68% acyanotic CHD) assessed by DASII, 48% and 12%
children had low ( £70)
Motor and Mental DQ, respectively.
The limitations of the present study include smaller
number of cyanotic CHD children, lack of non-CHD controls, lesser number
of patients in the older age-groups, and absence of follow-up after
surgery/control of cardiac failure. There are other psychosocial factors
that may have a negative impact on these children including physical
restriction, parental overprotection, school absence, and decreased
peer-interaction, which were not studied.
Nearly half the children (57.3%) had weight <-3 SD of
WHO growth chart. Malnutrition in infants with CHD is related to
increased energy expenditure and inadequate caloric intake for growth
[9]. Mean motor DQ was decreasing with the severity, which was in
accordance to previous studies [10] stating that developmental delay
increases with the complexity of heart disease. Stratification based on
risk for developmental delay has been given by American Heart
Association [4]. We found low DQ in high risk groups in all domains
compared to moderate- and low-risk groups.
More developmental delay was found in CCHD group in
various previous studies due to chronic hypoxia caused by underlying CHD
[7]. In a previous study [11] of neurodevelopmental status of newborns
and infants with congenital heart disease before and after open heart
surgery, newborns with acyanotic defect were more likely to demonstrate
neurologic abnormality than those with cyanotic defect. In another study
[12] from Canada, gross and/or fine motor delay was documented in 42%,
and 23% had global developmental delay. Higher number of children with
developmental delay in our study compared to these studies could be due
to the high prevalence of uncontrolled CHF; anemia and rickets may also
contri-bute to the same. Similar to our study, others have also found
that motor delay is more than mental delay among children with CHD
[13,14].
The high rate of developmental delay among children
with CHD demonstrated in this study has important implications for
practice and research. Future studies need to identify modifiable
factors affecting development among these group of children in addition
to those previously identified (e.g., congestive cardiac failure
and anemia). Screening and evaluation of developmental delay in
pediatric CHD population are essential steps to guide appropriate
interventions to maximize their overall development.
Contributors: DM: conceived and planned
the study, and supervised the conduct of the study and preparation of
the manuscript. KL: enrolled subjects, did the neurodevelopmental
assessment, analyzed data, and prepared the initial draft of the
manuscript. VM: echocardiographic studies. MJ: supervised the
neurodevelopmental assessment. VM and MJ: assisted in the planning of
the study and preparation of the manuscript. All authors approved the
final manuscript for publication.
Funding: None; Competing interest:
None stated.
|
What This Study Adds?
•
Developmental delay is common among children with congenital
heart diseases.
•
Developmental delay it is more common in those with cyanotic
heart disease, and in the motor domain.
|
References
1. Ferry PC. Neurologic sequelae of cardiac surgery
in children. Am J Dis Child. 1987;141:309-12.
2. Ferry PC. Neurologic sequelae of open-heart
surgery in children. An ‘irritating question’. Am J Dis Child.
1990;144:369-73
3. Bloom AA, Wright JA, Morris RD, Campbell RM,
Krawiecki NS. Additive impact of in-hospital cardiac arrest on the
functioning of children with heart disease. Pediatrics. 1997;99: 390-8.
4. Marino BS, Lipkin PH, Newburger JW, Peacock G,
Gerdes M, Gaynor JW, et al. Neurodevelopmental Outcomes in
Children With Congenital Heart Disease: Evaluation and Management: A
Scientific Statement From the American Heart Association. Circulation.
2012;126:1143-72.
5. Sharma R, Choudhary SK, Mohan MR, Padma MV, Jain
S, Bhardwaj M, et al. Neurological evaluation and intelligence
testing in the child with operated congenital heart disease. Ann Thorac
Surg. 2000;70:575-81.
6. Phatak P. Developmental Assessment Scale of Indian
Infants (DASII) – Revised Baroda Norms Manual, 1997.
7. Hoffman JI, Kaplan S. The incidence of congenital
heart disease. J Am Coll Cardiol. 2002;39:1890-900.
8. Juneja M, Jain R, Chakrabarty B, Mishra D, Saboo
P. Indian children with developmental disabilities: Early versus late
referral for intervention. Indian J Pediatr. 2014;81:1177-81.
9. Wernovsky G. Current insights regarding
neurological and developmental abnormalities in children and young
adults with complex congenital cardiac disease. Cardiol Young. 2006; 16
(Suppl. 1):92-104.
10. Donofrio MT, Bremer YA, Schieken RM, Gennings C,
Morton LD, Eidem BW, et al. Autoregulation of cerebral blood flow
in fetuses with congenital heart disease: the brain sparing effect.
Pediatr Cardiol. 2003;24:436-43.
11. Limperopoulos C, Majnemer A, Shevell MI,
Rosenblatt B, Rohlicek C, Tchervenkov C. Neurodevelopmental status of
newborns and infants with congenital heart defects before and after open
heart surgery. J Pediatr. 2000;137:638-45.
12. Limperopoulos, Majnemer A, Shevell MI, Rosenblatt
B, Rohlicek C, Rosenblatt B. Predictors of developmental disabilities
after open heart surgery. J Pediatr. 2002;139:51-8.
13. Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J,
Gerdes M, Zackai E, et al. Patient characteristics are important
determinants of neurodevelopmental outcome at one year of age after
neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg.
2007;133:1344-53.
14. Nasiruzzamarrt AHM, Hussain MZ, Baki MA, Tayeba
MA, Mollah MN. Growth and developmental status of children with
congenital heart disease. Bangladesh Med J. 2011;40:54-7. Available
from: www.banglajol.info/index. php/BMJ/article/ viewFile/18512/12967.
Accessed May 2, 2015.
|
|
|
 |
|

