|
|
|
Indian Pediatr 2014;51: 900-902 |
 |
Nasal Intermittent Positive Pressure
Ventilation with Heliox in Premature Infants with Respiratory
Distress Syndrome: A Randomized Controlled Trial
|
|
Xue Li, Jie Shen, Jinlin Zhao, Shifang Tang and Yuan Shi
From the Department of Pediatrics, Daping Hospital,
Third Military Medical University, Chongqing, China.
Correspondence to: Dr Yuan Shi, Director and
Professor, Department of Pediatrics, Daping Hospital, Third Military
Medical University, Chongqing, 400 042, China.
Email: [email protected]
Received: March 27, 2014;
Initial review: April 28, 2014;
Accepted: September 02, 2014.
|
Objective: To assess the efficacy of nasal intermittent positive
pressure ventilation with heliox in preterm infants with respiratory
distress syndrome.
Methods: Premature infants
with mild respiratory distress syndrome requiring non-invasive
respiratory support were eligible. Infants were randomly assigned to
heliox or air-oxygen group. The main outcome was the length of
ventilation.
Results: Heliox significantly
decreased the length of ventilation. The length of ventilation was
positively correlated with interleukin-6 at baseline. Carbon dioxide
elimination was better in the heliox group.
Conclusion: Heliox delivered
with nasal intermittent positive pressure ventilation may be
effective in reducing length of ventilation and increasing carbon
dioxide elimination.
Keywords: Helium, Oxygen, Prematurity,
Ventilation.
|
|
The use of heliox for neonatal ventilation has
gained interest in recent years [1]. Its beneficial effect lies in the
lower density compared to air-oxygen mixture (airox) [2], thereby
reducing the driving pressure needed under turbulent flow conditions and
promoting laminar flow in areas of airway narrowing [3]. These physical
properties have been reported to be beneficial in different neonatal
diseases [4]. To the best of our knowledge, there have been no reports
about nasal intermittent positive pressure ventilation (NIPPV) with
heliox in neonates. The aim of our study was to assess the efficacy of
NIPPV with heliox on length of ventilation and lung inflammation
cytokines in preterm infants with respiratory distress syndrome (RDS).
Methods
Neonates <37 weeks of gestation with a diagnosis of
RDS who required a fraction of inspired oxygen (FiO 2)
³0.3 to
maintain PaO2 >50 mmHg in
the first hour after birth were eligible for enrolment in this
randomized controlled trial. The diagnosis of RDS was based on clinical
manifestations and chest radiograph findings. All the neonates had
bedside chest X-ray done by the same machine after admission to
neonatal intensive care unit (NICU). Infants were excluded from this
study if they met any of the following criteria: pneumonia, meconium
aspiration, major congenital anomalies, intubation in the delivery room,
transient tachypnea without radiological evidence of RDS, consent not
provided or refused, or severe respiratory failure requiring intubation.
Participants were randomly assigned to receive
helium-oxygen (heliox) or air-oxygen (airox) mixture using a
sealed-envelope method. Heliox group was treated with NIPPV for 3 hours
with heliox (70% helium and 30% oxygen) delivered from cylinders
followed by airox until NIPPV was no longer needed. The airox group
received NIPPV with (30% oxygen and 70% air). The main outcome measures
were length of ventilation (time taken to successful extubation from
ventilation), and maintaining oxygen saturation >90%. The physicians
were unmasked as heliox was delivered by special cylinders. Infants were
considered for weaning from nasal respiratory support when peak
inspiratory pressure was below 20 cmH 2O
and FiO2 was below 25%.
Infants were intubated when pH <7.2, PaO2
>50 mmHg with FiO2 >0.5,
PaCO2 >60 mmHg or having
frequent episodes of apnea. The secondary outcomes were changes in
transcutaneous pressure of oxygen and carbon dioxide (TcPO2
and TcPCO2), lung
inflammation cytokines, intubation rate and complications.
All the data were analyzed using SPSS 17 software.
Fisher’s exact test, 2-way analysis of variance with repeated measures,
multiple linear regression, correlation analysis and independent-samples
t test were used to analyze the data.
The study was approved by the ethics committee of
Daping Hospital. Informed consent was obtained from parents before
enrolment of their children into the study. The sample size of 32
participants was calculated to detect a reduction of 0.8-day in the
length of ventilation with 80% power for a 2-sided
a of 0.05.
Results
Thirty-six neonates were included; 19 were randomized
to heliox group and 17 to control group. The clinical characteristics
are compared in Table I.
TABLE I Baseline Characteristics of Infants in The Study
|
Heliox
(n=19)
|
Airox
(n=17)
|
|
Males, n (%)
|
13 (68.4) |
10 (58.8) |
|
Birth weight, mean (SD), kg |
2.15 (0.47) |
2.19 (0.44) |
|
Gestational age, mean (SD), wk |
34.2 (1.8) |
34.3 (1.8) |
|
Cesarean delivery, n (%)
|
13 (68.4) |
14 (82.4) |
|
Antenatal steroids, n (%)
|
11(57.9) |
9 (52.9) |
|
Apgar 1 min, median (range) |
9 (6-10) |
9 (7-10) |
|
Stage of X-ray chest, median (range) |
1.4 (1-3) |
1.8 (1-3) |
|
7 (36.8) |
8 (47.1) |
|
Need of surfactant, n (%) |
2 (10.5) |
2 (11.8) |
|
Birth weight <1500g, n (%) |
2 (11.1) |
2 (11.8) |
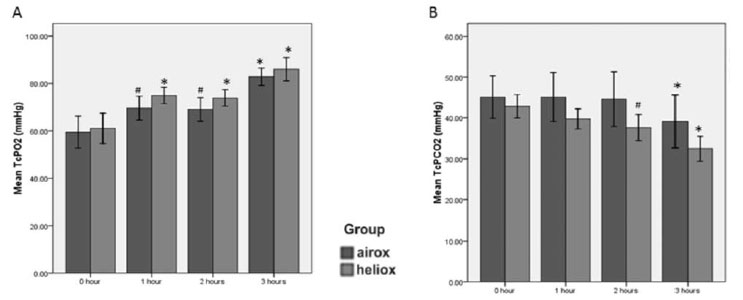
Heliox significantly reduced mean (SD) length of
ventilation in comparison to airox. Length of ventilation was
significantly and positively correlated with IL-6 at baseline (r=0.474,
P=0.006). Three infants required intubation in the airox group,
while none required it in the heliox group. Both TcPO 2
and TcPCO2
improved after 3 hours in each group (P<0.001);
the difference was not statistically significant at each time point (Fig.
1). Carbon dioxide elimination was better in the heliox group (10.4
mmHg vs. 6.0 mmHg, P=0.03) (Fig. 1).
 |
|
Fig. 1 Comparion of transcutaneous
pressure of oxygen (TcPO 2) and
carbon dioxide (Tc PCO2) in neonates receiving Heliox or Airox.
|
Cytokines were not significantly different between
the two groups, except for IL-6, a reduction that was lower in the
heliox group (Table II). Seven patients in the heliox
group and five in the control group were diagnosed with patent ductus
arteriosus (PDA), while 3 patients in the heliox group and 1 patient in
the control group were diagnosed with necrotizing enterocolitis (NEC).
After 3 hours, there was no statistically significant differences of
peak inspiratory pressure, mean airway pressure, oxygen saturation and
respiratory rate in the two groups.
TABLE II Comparison of Outcomes Between Neonates Receiving Heliox or Airox for Ventilation
|
Heliox (n=19) |
Controls (n=17) |
P |
|
Length of ventilation, h
|
39.3 (15.1) |
57.8 (25.0) |
0.02 |
|
Interleukin-6, u/mL |
48.4 (48.3) |
146.4 (51.2) |
0.17 |
|
*Malonyldialdehyde
|
6.4 (2.8) |
5.1 (3.6) |
0.25 |
|
#Tumor necrosis factor-a |
347.4 (340.7) |
296.5 (281.9) |
0.67 |
|
Myeloperoxidase, IU/L |
235.9 (233.9) |
168.9 (166.9) |
0.38 |
|
Inducible nitric oxide synthase, IU/mL |
10.7 (7.9) |
9.6 (7.4) |
0.70 |
|
Interleukin-6 was collected at
baseline and 3 hours of the adminis-tration, while others were
collected at 3 hours of the administration. All data in mean
(SD); *mg/mL; # ng/L. |
Discussion
In our study, heliox decreased length of ventilation
in comparion to airox. Both TcPO2
and TcPCO2
improved after 3 hours, and carbon dioxide elimination was better in the
heliox group. Analysis of lung inflammation cytokines showed no
statistically significance between the two groups, but the values of
IL-6 showed a noticeable reduction in the heliox group.
The technique was well tolerated in all infants. The
small sample size, short time frame of heliox ventilation and unmasked
assignement were the main limitations of this study. Our study findings
of reduction in lenth of ventilation were similar to those by Elleau,
et al. [6]. However, Colnaghi, et al. [7] found heliox failed
to reduce length of ventilation when combined with nasal CPAP. The
delivery method of heliox is crucial to its efficacy [8]. NIPPV might
have increased the efficacy of heliox as compared with nasal CPAP.
Helium might have anti-inflammatory effects [9,10]; heliox with NIPPV
might have alleviated inflammation reaction in the acute phase of RDS.
Heliox group in our study showed a better elimination of carbon dioxide,
which is in agreement with previous reports [11,12]. This might be
attributed to the better carbon dioxide diffusion in heliox [13,14].
We conclude that heliox delivered with NIPPV may be
effective in reducing length of ventilation and increasing carbon
dioxide elimination in
preterm neonates with mild RDS. Large, double-blind, randomized
controlled trials are needed to assess further benefits of heliox
therapy in preterm infants.
Contributors: XL, LI: conceptualized and designed
the study, drafted the initial manuscript, and approved the final
manuscript as submitted; JS: designed the data collection instruments,
and coordinated and supervised data collection, critically reviewed the
manuscript; JZ: carried out the initial analyses, reviewed and revised
the manuscript and approved the final manuscript as submitted; ST:
carried out the initial analyses, reviewed and revised the manuscript
and approved the final manuscript as submitted; YS: instructed the
designation and implementation of the study, reviewed and revised the
manuscript and approved the final manuscript as submitted.
Funding: None; Competing interests: None
stated.
|
What This Study Adds?
•
NIPPV with heliox reduces length
of ventilation and increases the carbon dioxide elimination in
preterm infants with mild respiratory distress syndrome.
|
References
1. Davis PG, Morley CJ, Owen LS. Non-invasive
respiratory support of preterm neonates with respiratory distress:
Continuous positive airway pressure and nasal intermittent positive
pressure ventilation. Semin Fetal Neonat Med. 2009;14:14-20.
2. Szczapa T, Gadzinowski J. Use of heliox in the
management of neonates with meconium aspiration syndrome. Neonatology.
2011;100:265-70.
3. Ozima M, Podosek FA. Noble Gas Geochemistry.
Cambridge University Press, 2002.
4. Papamoschou D. Theoretical validation of the
respiratory benefits of helium-oxygen mixtures. Resp Physiol.
1995;99:183-90.
5. Dani C, Fontanelli G, Lori I, Favelli F, Poggi C.
Heliox non-invasive ventilation for preventing extubation failure in
preterm infants. J Matern-Fetal Neonat Med. 2013;26:603-7.
6. Elleau C, Galperine R, Guenard H, Demarquez JL.
Helium-oxygemixture in respiratory distress syndrome: A double-blind
study. J Pediatr. 1993;122:132-6.
7. Colnaghi M, Pierro M, Migliori C, Ciralli F,
Matassa PG, Vendettuoli V, et al. Nasal continuous positive
airway pressure with heliox in preterm infants with respiratory distress
syndrome. Pediatrics. 2012;129:e333-8.
8. Chowdhury MM, McKenzie SA, Pearson CC, Carr S, Pao
C, Shah AR, et al. Heliox therapy in bronchiolitis: Phase III
multicenter double-blind randomized controlled trial. Pediatrics.
2013;131:661-9.
9. Nawab US, Touch SM, Irwin-Sherman T, Blackson TJ,
Greenspan JS, Zhu G, et al. Heliox attenuates lung inflammation
and structural alterations in acute lung injury. Pediatr Pulmonal.
2005;40:524-32.
10. Yilmaz S, Daglioglu K, Yildizdas D, Bayram I,
Gumurdulu D, Polat S. The effectiveness of heliox in acute respiratory
distress syndrome. Ann Thorac Med. 2013;8:46-52.
11. Dani C, Fontanelli G, Lori I, Favelli F, Poggi C.
Heliox non-invasive ventilation for preventing extubation failure in
preterm infants. J Matern-Fetal Neonat Med. 2013;26:603-7.
12. Migliori C, Gancia P, Garzoli E, Spinoni V,
Chirico G. The Effects of helium/oxygen mixture (heliox) before and
after extubation in long-term mechanically ventilated very low birth
weight infants. Pediatrics. 2009;123:1524-8.
13. Gupta VK, Cheifetz IM. Heliox administration in
the pediatric intensive care unit: an evidence-based review. Pediatr
Crit Care Med. 2005;6:204-11.
14. Abd-Allah SA, Rogers MS, Terry M, Gross M, Perkin
RM. Helium-oxygen therapy for pediatric acute severe asthma requiring
mechanical ventilation. Pediatr Crit Care Med. 2003;4:353-7.
|
|
|
 |
|

