|
|
|
Indian Pediatr 2012;49: 897 -910 |
 |
Consensus Guidelines on Evaluation and
Management of Suspected Acute Viral Encephalitis in Children in
India
|
|
*Suvasini Sharma,
‡Devendra
Mishra, *Satinder Aneja, #Rashmi
Kumar, ^Amita Jain,
$Vipin M Vashishtha
for the Expert Group on Encephalitis, Indian Academy of Pediatrics.
From *Department of Pediatrics, Lady Hardinge Medical
College, Delhi; ‡Department of Pediatrics, Maulana Azad Medical College,
Delhi; Departments of #Pediatrics and ^Microbiology, King George’s
Medical University, Lucknow, UP; and
$Mangla Hospital & Research Center, Shakti Chowk, Bijnor, Uttar Pradesh;
India.
Correspondence to: Dr Devendra Mishra, Associate
Professor, Department of Pediatrics, Maulana Azad Medical College,
Delhi 110 002.
Email: drdmishra@gmail.com
|
|
Justification: Viral encephalitis
is an important cause of mortality and morbidity in children. The
etiological agents are varied, and physicians treating such children
often feel limited by the lack of uniform guidelines on evaluation and
management of these critically ill children in resource-constrained
settings.
Process: An ‘Expert Group Meeting
on Viral Encephalitis in Children’ was held on 19th January, 2012 in
Gurgaon, Haryana (under the aegis of PEDICON 2012, the National
Conference of Indian Academy of Pediatrics). The invited experts
included pediatricians and microbiologists with expertise in the
relevant field. Various issues related to the subject were discussed and
it was decided to bring out recommendations on the topic. The final
recommendations were produced after circulating the draft document, and
incorporating/discussing all changes, by e-mail.
Objectives: To aid the
pediatrician in the evaluation and management of children with suspected
viral encephalitis and to assist the public health authorities in acute
encephalitis surveillance. These guidelines do not cover viral
encephalitis in the neonatal period and in immunocompromised children,
Rabies encephalitis, and chronic viral encephalitis such as Subacute
sclerosing panencephalitis (SSPE).
Recommendations: Recommendation
for evaluation and management of suspected viral encephalitis in
children are presented. In any acute encephalitis outbreak,
pediatricians should be aware of the common viral causes of encephalitis
in their area, what information and samples they should collect, and the
contact details of the District Surveillance Unit. Pending specific
diagnosis and therapy (which may or may not be possible), prompt
empirical therapy and meticulous supportive care are important to
prevent ongoing brain damage, and improve outcome.
Key words: Child, Encephalitis, Guidelines,
India, Investigations, Management.
|
|
Viral encephalitis is an important cause of
mortality and morbidity in children. It may be sporadic like herpes
simplex encephalitis(HSE), or epidemic such as Japanese B encephalitis
(JE). The etiological agents are varied, and physicians treating such
children often feel limited by the lack of availability of diagnostic
testing for most of these agents. There are numerous lacunae in our
knowledge, problems in epidemiological investigations, lack of
diagnostic facilities, as well as difficulties in managing these
critically ill children in smaller centers in our country (Box1).
Pediatricians who treat these children should be aware of how to manage
a child with suspected encephalitis, as specific antiviral therapy is
lifesaving in some diseases and these should be diagnosed without delay.
Moreover, optimum supportive care is of paramount importance in the
management of these children. These guidelines have been developed to
aid the pediatrician in the management of children with suspected viral
encephalitis, in both sporadic and epidemic settings in India. These
guidelines do not cover viral encephalitis in the neonatal period and in
immuno-compromised children, Rabies encephalitis, and chronic viral
encephalitis such as Sub-acute sclerosing pan-encephalitis (SSPE).
|
Box 1 Problems Encountered in the Management of Children
With Suspected Viral Encephalitis
• Paucity of data about the regional
epidemiology and etiology of viral encephalitis
• Lack of easily available, low-cost
microbiological testing for agents of viral encephalitis
• Lack of specific treatments for majority of
the etiological agents
• High incidence of mimickers - pyogenic
meningitis, cerebral malaria, tubercular meningitis, acute
desseminated encephaloyelitis etc.
• Lack of facilities for intensive care in
the periphery
• Lack of facilities for neuroimaging in the
periphery.
• Inappropriate response during epidemics -
what samples to take, how to store, whom to inform, etc.
• Patient delay in seeking health care
• Delay/not performing lumbar punctures
• Inappropriate supportive care
|
Process
An ‘Expert Group Meeting on Viral Encephalitis in
Children’ was held on 19th
January, 2012 in Gurgaon, Haryana (under the aegis of PEDICON 2012, the
National Conference of Indian Academy of Pediatrics). The invited
experts included pediatricians and microbiologists with expertise in the
relevant field (Annexure I). Participants had been
previously allotted specific topics for review. During the meeting, the
problems related to managing these critically ill children in
resource-constrained settings were identified (Box 1).
Subsequently, the experts deliberated on evaluation and management
issues and a consensus reached on contentious topics. At the end of the
meeting, it was decided to bring out recommendations on evaluation and
management of suspected viral encephalitis in children, and a writing
group identified for the purpose. Due to the lack of country-specific
epidemiological information on the relative contribution of various
etiologies to the burden of viral encephalitis, it was decided not to
categorize the recommendations by either ‘level of evidence’ or
‘strength of recommendation’. The draft was circulated by e-mail among
all experts, and after incorporating all suggestions and review, the
final document was produced.
Epidemiology and Disease Burden
Definitions
The various definitions used in the document have
been delineated in Box 2. Encephalopathy may be caused by
many diverse causes including, systemic infection, metabolic
derangement, inherited metabolic disorders, toxins, hypoxia, trauma,
vasculitis, and central nervous system infection. Encephalitis means
inflammation of the brain, which is difficult to decipher clinically and
therefore, surrogate clinical markers are often used, including
inflammatory changes in the cerebrospinal fluid or parenchymal
inflammation on imaging [1]. Causes include viruses, small intracellular
bacteria that directly infect the brain parenchyma and some parasites.
It can also occur without direct brain infection, for example in acute
disseminated encephalomyelitis (ADEM), or antibody-associated
encephalitis. Acute encephalitis syndrome (AES) is a term used by WHO
for syndromic surveillance in the context of Japanese encephalitis (JE)
[2]. This definition includes not only viral encephalitis, but also all
etiologies of fever and altered sensorium, such as bacterial meningitis,
tubercular meningitis, cerebral malaria, and acute disseminated
encephalomyelitis. Moreover, the duration of illness to classify as
‘acute’, has also not been clarified. After much discussion, a period of
up to 14 days was considered by consensus to define ‘acute’. Although
the expert group felt this definition had problems and seemed
complicated, and alternative terms such as ‘acute febrile
encephalopathy’ and ‘acute encephalitis-like syndrome’ were considered,
it was ultimately decided to continue with this definition for the sake
of uniformity. Case definitions of suspected, probable and confirmed JE
have previously been provided by the WHO [2].
|
Box 2 Important Definitions
Encephalopathy
Encephalopathy describes a clinical syndrome
of altered mental status, manifesting as reduced consciousness
or altered behavior [1].
Encephalitis
Encephalitis means inflammation of the brain.
It is strictly a pathological diagnosis; but
surrogate clinical/imaging markers may provide evidence of
inflammation.
Acute Encephalitis Syndrome*
Clinically, a case of acute encephalitis
syndrome is defined as a person of any age, at any time of year
with the acute onset of fever and a change in mental status
(including symptoms such as confusion, disorientation, coma, or
inability to talk) AND/OR new onset of seizures (excluding
simple febrile seizures) [2].
Japanese B encephalitis (JE)
Laboratory-confirmed JE: A suspected case
that has been laboratory-confirmed as JE [2].
Probable JE: A suspected case that occurs
in close geographic and temporal relationship to a
laboratory-confirmed case of JE, in the context of an outbreak
[2].
|
|
* This definition includes not only
viral encephalitis, but also all etiologies of fever and altered
sensorium, such as bacterial meningitis, tubercular meningitis,
cerebral malaria, acute disseminated encephalomyelitis etc.
Other early clinical findings may include an increase in
irritability, somnolence or abnormal behavior greater than that
seen with usual febrile illness.
|
Etiological Agents
Viral agents that have the potential of infecting the
central nervous system in humans have previously been detailed [3]; the
common causes of viral encephalitis reported from India are listed in
Box 3. Agents that may be encountered in AES in an epidemic
form include Japanese encephalitis, which is a major public health
problem because of large endemic areas in the country, the high case
fatality rate (20-30%) and frequent residual neuropsychiatric damage
(50-70%) [2]; Enteroviruses, especially EV 71 [4], reported also from
sporadic encephalitis cases [5]; Chandipura virus [6,7]; Nipah virus
[8]; and, Chikangunya virus [9]. Another common viral agent of AES in
the epidemic setting, being recognized more commonly now, is Dengue
virus [10].
|
BOX 3 Agents of
Clinically Important Viral Encephalitis in India*
Japanese encephalitis virus
Enteroviruses
• Outbreak-2006, east UP (EV
89,76); 2008, Lucknow (EV 71)
• Sporadic-2004-06, AMU, UP
(EV 71); 2007, Delhi (EV 71)
Herpes simplex virus 1 (HSV-1)
Dengue Virus (encephalopathy)
Measles virus
Chandipura
• Outbreak-Andhra Pradesh
2003; Gujarat 2004; Nagpur 2005; Nagpur 2007
• Sporadic-2005-06; Andhra
Pradesh
Mumps virus
Chikungunya
Varicella zoster virus (VZV)
Epstein-Barr virus (EBV)
Human immunodeficiency virus (HIV)
Human herpesvirus 6 (HHV-6)
Nipah (Handra)
• Outbreak: 2001, Siliguri;
2007, West Bengal
West Nile virus#
Kyasanur Forest Disease
Rabies
|
|
*Cerebral
malaria, pyogenic/tubercular meningitis and rickettsial diseases
may mimic the clinical and/or laboratory characteristics of
these agents, and may need to be excluded by appropriate tests;
# Unconfirmed reports of recent
outbreak in Kerala in October, 2011.
|
Viral agents responsible for sporadic encephalitis
include Varicella zoster virus, Mumps, Human herpes virus 6 and 7,
Epstein Barr virus, and most importantly, Herpes simplex virus.
Herpse simplex virus encephalctis (HSE) is the most common cause of
sporadic fatal viral encephalitis, with an incidence of 1-3/million in
western countries [11] Not much information is available regarding
proportion of AES cases due to HSE in the Indian setting. In untreated
patients, mortality is high (70%), which is decreased to 30% in treated
patients (risk of sequelae of around 11%) [12]. Measles virus can cause
acute encephalitis and has frequently been implicated in epidemic
encephalitis, sometimes without rash [13]; although the evidence has
been questioned [14,15].
Emerging Viral Agents and Changing Epidemiology
The changing epidemiology and newer viral agents
causing AES worldwide have recently been reviewed [16,17]. Various other
viral agents e.g., Human Parvovirus 4 [18], West Nile virus [19,20],
Bagaza virus [21], Coxsackie virus [22] have been reported in sporadic
AES cases from India. Various non-viral causes associated with
encephalitis were recently described [23]. Some authors have also
reported epidemic-like occurrence of AES due to non-infective causes in
children from India e.g., plant toxins (Cassia occidentalis)
[24], heat stroke [25], and Reye’s syndrome [26-28]. The exact
epidemiologic significance of some of these reports is difficult to
elucidate from the available literature.
Evaluation and Management
Acute encephalitis syndrome is a medical and
neurological emergency, requiring immediate conside-ration of key issues
including immediate life support, identification of cause, and when
available, institution of specific therapy. Management guidelines at the
community level for a child with features suggestive of
meningoencephalitis have previously been provided by PATH: Japanese
Encephalitis Clinical Care Guidelines, 2005 [29], and by UNICEF and
Government of India (Facility-based IMNCI Participants’ Manual [30]. Our
guidelines reiterate the previously detailed initial stabilization and
supportive management of a child with altered sensorium, and provide
additional information on evaluation and management. The evaluation
(clinical as well as investigations) and treatment have to proceed
simultaneously (Box 4). A step-wise management is
described.
|
Box 4 Evaluation and Management of a
Child with Acute Encephalitis Syndrome
Step I: Rapid assessment and
stabilization
• Establish and maintain
airway: Intubate if GCS<8,
impaired airway reflexes, abnormal respiratory pattern, signs of
raised ICP, oxygen saturation <92% despite high flow oxygen, and
fluid refractory shock
• Ventilation, Oxygenation
• Circulation: Establish IV
access, take samples (CBC, Blood sugar, KFT, LFT, electrolytes,
blood gas, lactate, PS and RDT for malarial parasite, serology
for viruses), Fluid bolus if in circulatory failure (20 mL/kg
NS), inotropes if required
• Identify signs of cerebral
herniation or raised ICP
• Temperature: treat fever
and hypothermia
• Treat ongoing seizures-
Benzodiazepine, followed by phenytoin loading
Step II: Clinical evaluation:
History and Examination
Step III:
Investigation/Samples to be collected
• CSF
• Blood/serum, Urine
• MRI (CT, if MRI not
available/possible), avoid sedation
• Throat swab, nasopharyngeal
swab
Step IV: Empirical Treatment
( must be started if CSF cannot be done/report will take time
and patient sick)
• Ceftriaxone
• Acyclovir (use in all
suspected sporadic viral encephalitis)
Artesunate (stop if
peripheral smear and RDT are negative)
Step V: Supportive care and
treatment
• Maintain euglycemia,
Control fever, Maintain hydration
• Treat raised intracranial
pressure, mild head-end elevation–15-30°
• Treat seizures; Give
anticonvulsant if history of seizures or if GCS <8, or child has
features of raised ICT
• Steroids: Pulse steroids (methylprednisolone
or dexamethasone) must be given in children with suspected ADEM.
Step VI: Prevention/treatment
of complications and rehabilitation
• Physiotherapy, posture
change, Prevent bed sores and exposure keratitis
• Complications: aspiration
pneumonia, nosocomial infections, coagulation disturbances
• Nutrition: early feeding
• Psychological support to patient and family
|
Step 1: Rapid Assessment and Stabilization
As in any emergency, initial steps should be directed
to ensuring adequacy of airway, breathing and circulatory function.
Airway management is of paramount importance in children with altered
states of consciousness, as their protective reflexes are obtunded and
they are more prone to aspiration. Children with Glasgow Coma Score less
than 8 should preferably be intubated; mechanical ventilation should be
provided in case the breathing efforts are not adequate. Appropriate
oxygenation should be ensured.
The next important step is establishment of vascular
access. If there is evidence of circulatory failure, fluid bolus (20 mL/kg-Normal
saline) should be administered. Samples should be drawn for various
investigations. If hypoglycemia is present, intravenous glucose should
be administered. If the child is having seizures, or there is history of
a seizure preceding the encephalopathy, anticonvulsant (intravenous
benzodiazepine followed by phenytoin loading 20 mg/kg) should be
administered [31]. If there are features of raised intracranial pressure
(asymmetric pupils, tonic posturing, papilledema, evidence of herniation),
measures to decrease intracranial pressure should be rapidly instituted
(head elevation, minimal disturbance, normothermia, pharmacotherapy,
hyperventilation, etc.). Acid base and electrolyte abnormalities should
be corrected. Normothermia should be maintained.
Step 2: Detailed History and Examination
A careful history should be taken with special
emphasis on onset and duration, and other features such as fever,
headache, vomiting, irritability, seizures, and rash (Box 5).
There may be a prodrome of upper respiratory illness, flu-like illness
or diarrhea. Recent history or contact with a child having chicken pox
or mumps must be enquired. The place of residence of the child (endemic
area for any disease e.g., JE), recent history of travel, or any
occurrence of similar illness in the neighborhood must be noted.
|
Box 5 Important Points in The History of a
Child With AES
• Fever, headache, vomiting,
seizures, abnormal posturing
• Altered behavior,
cognition, personality changes, altered consciousness
• Prodromal symptoms-
flu-like illness, diarrhea
• Rash, vesicles, past
history of chicken pox
• Residence of child:
Rural/urban, endemic for cerebral malaria, any epidemic of AES
in neighborhood
• History of animal contact,
insect bite, dog bite
• Drug or toxin exposure-
enquire for presence of any drugs at home
• Recent history of travel
• History of trauma
• Personal or family history
of seizure disorder
• Recent immunizations
• History of recurrent
episodes of encephalopathy: These are characteristic of some
inborn errors of metabolism (urea cycle defects, organic
acidemias and fatty acid oxidation defects), but may also be
present in migraine, epilepsy, substance abuse, and Munchausen
syndrome by proxy
• Other concurrent systemic
illness e.g. jaundice (hepatic failure), pneumonia (hypoxic
encephalopathy), diarrhea (dyselectrolytemia), dysentery (shigella
encephalopathy)
• Past medical illness:
Diabetes, congenital heart disease, chronic kidney or liver
disease
• Family history of previous
infant/child deaths
• Pre-morbid developmental/
neurological status of the child
• Risk factors for immunodeficiency- HIV risk
factors, cancer treatment, steroid/immunosuppressant treatment
|
A history of fever or recent illness suggests an
acute infectious etiology, but other disorders in which encephalopathy
maybe preceded by a febrile illness must also be considered. These
include acute disseminated encephalomyelitis, Reye’s syndrome, and
mitochondrial and other inborn errors of metabolism [32]. History of
trauma, drug/toxin exposure, dog bite, past medical illnesses, and
family history must be elicited. Past history of similar illness may
indicate the presence of an underlying inborn error of metabolism.
Encephalitis associated with gastrointestinal symptoms include
infections with enteroviruses, rotavirus and human parechovirus [1].
Encephalitis associated with respiratory illnesses may be due to
influenza viruses, paramyxoviruses and the bacteria, Mycoplasma
pneumoniae [1]; those with influenza associated encephalopathy may,
in addition, have associated myositis [33].
The general physical examination may provide helpful
etiological clues. Presence of pallor may indicate cerebral malaria, or
intracranial bleed. Icterus could indicate leptospirosis, hepatic
encephalopathy, or cerebral malaria. Skin rashes are common in
meningococcemia, dengue, measles, varicella, rickettsial diseases,
arboviral diseases, and enteroviral encephalitis. Petechiae are seen in
meningococcemia, dengue and viral hemorrhagic fevers. Parotid swelling
and orchitis point towards mumps as etiology. Mumps encephalitis, may,
however, occur without parotitis [34]. In a study of 137 patients with
mumps meningitis, parotitis was detected only in 37% of patients [35].
Labial herpes in young children may point towards herpes simplex virus
encephalitis [36].
The neurological examination is targeted to document
the level and localization of brain dysfunction. It may also provide
information about the potential causes. The level of consciousness must
be recorded in the form of an objective scale, such as the Glasgow Coma
Scale (GCS). A modified GCS should be used for infants and young
children [37]. While the GCS allows efficient, standardized
communication of a child’s state, a more detailed description of the
child’s clinical findings is often more useful for relaying detailed
information and detecting changes over time.
Pupillary size, shape, symmetry and response to light
provide valuable clues to brainstem and third nerve dysfunction. Topical
administration of mydriatics must be avoided, but if done, should be
documented to avoid confusion in interpretation. Unilateral pupillary
dilatation in the comatose patient should be considered as evidence of
oculomotor nerve compression from ipsilateral uncal herniation, unless
proved otherwise [38]. Symptoms of progressive symmetrical external
ophthalmoplegia suggest Bickerstaff brainstem encephalitis in
association with M. pneumoniae, and can serve as a clue to the
diagnosis, especially when associated with ataxia [39].
In HSE, neurological findings are mostly related to
dysfunction of the fronto-temporal lobes viz., personality
changes, confusion and disorientation. However, absence of herpes
labialis, focal seizures or unilateral neurological findings does not
rule out HSE. CT is usually normal in first 4-6 days of the disease
[11]. MRI demonstrates high signal intensity lesions on T2-weighted,
diffusion-weighted, and FLAIR images earlier in the course [13]. The MRI
may rarely be normal in HSE. The optimum chance of obtaining a positive
CSF PCR in HSE is between 2-10 days after the onset of illness [31].
The presence of oculocephalic (doll’s eye),
oculovestibular, corneal, cough and gag reflexes must be looked for to
check brainstem function. Brainstem dysfunction is an important feature
in some causes of viral encephalitis such as enterovirus 71, mumps, and
rabies [1]. The trunk, limb position, spontaneous movements, and
response to stimulation must be observed to look for any focal deficits,
and posturing (decerebrate or decorticate). The power of the limbs and
deep tendon reflexes must be checked. Brain tissue deforms
intra-cranially and moves from higher to lower pressure when there is
asymmetric, or generalized increased intracranial pressure [40]. This
gives rise to the various herniation syndromes. Special attention should
be given to posturing because it often signals a brainstem herniation
syndrome [41]. The importance lies in recognition and prompt treatment,
before the damage becomes irreversible. Associated acute flaccid
paralysis along with encephalitis can be seen in enterovirus infections
(anterior horn cell involvement), poliomyelitis (anterior horn cell
involvement), acute disseminated encephalomyelitis (due to myelitis) and
rarely in JE. The patient should be carefully observed for the presence
of subtle seizures (twitching of fingers, mouth, eyelid etc). Myoclonic
jerks are seen frequently in enterovirus encephalitis [42]. Dystonia or
extrapyramidal movements signify extrapyramidal involvement which
is very common in JE, seen in up to 1/3 rd
of children (43). Fundus examination must be performed to look for
papilledema and retinal hemorrhages. Retinal hemorrhages are an
important clue for cerebral malaria in endemic setting, being present in
nearly a quarter of the patients [44]. Presence of signs of
meningeal irritation (neck rigidity, Kernig’s sign and Brudzinski’s
sign) must be looked for. Systemic examination must be performed to look
for hepatosplenomegaly, pulmonary involvement such as pneumonia, pleural
effusions, and cardiac involvement such as myocarditis. Myocarditis is
an important complication of EV 71 encephalitis [42]. Children with EV
71 encephalitis may also develop neurogenic pulmonary edema.
Step 3: Investigations
Basic investigations: Basic blood investigations
which should be obtained in all patients with AES include a complete
blood count (including platelet count), blood glucose, serum
electrolytes, liver and kidney function tests, blood culture, arterial
blood gas, and lactate (if available). A peripheral smear for malarial
parasite and rapid diagnostic test for malaria should be obtained. A
chest X-ray should also be obtained.
Lumbar puncture: If the patient is
hemodynamically stable, and no features of raised intracranial pressure,
a lumbar puncture should be performed. If lumbar puncture is
contraindicated, a neuroimaging study should be obtained prior to the
lumbar puncture. Empirical treatment (Step 4) should be started pending
the results of lumbar puncture and/or neuroimaging studies.
The CSF analysis is an important investigation in
children with AES. CSF should be examined for cytology, biochemistry,
gram stain, Ziehl-Nielsen stain for acid fast bacilli, bacterial
culture, latex agglutination, PCR for HSV 1 and 2, and IgM antibodies
for JE and for Dengue virus (if suspected). Concurrent blood sugar must
also be measured to look for the CSF to blood sugar ratio. 1-2 mL CSF
should be stored for other virological studies, if needed. Usual CSF
findings in viral encephalitis include lymphocytic pleocytosis, mild to
moderately elevated protein, and normal CSF sugar. Similar findings may
occur in tubercular meningitis and partially treated pyogenic
meningitis; however, the CSF sugar is likely to be low in these
situations.
Neuroimaging: Only CT scan may be possible in the
emergency situation but it may give valuable information such as
presence of bleed, cerebral edema, temporal lobe hypodensities in herpes
simplex encephalitis, thalamic abnormalities in JE, and basal exudates
and hydrocephalus in tubercular meningitis. CT may also show brain
herniation, effacement of cisterns, and infective collections such as
brain abscesses and subdural empyema. If possible, an MRI should be
obtained, as soon as the patient is stable. MRI is not needed if the
etiology is clear by other investigations e.g., cerebral malaria,
pyogenic meningitis; or if suggestive changes are seen on CT; or in
epidemic situations where the likely etiology is already known. In all
other patients, MRI provides useful information regarding the etiology
and alternative diagnoses. However, the availability, cost, and
difficulties in transporting sick and unstable patients for MRI may be
limiting factors. MRI sequences must include diffusion weighted imaging
to detect early changes, and a gadolinium enhanced study.
Suggestive MRI findings are present in some
etiologies of viral encephalitis such as Herpes simplex encephalitis,
JE, enterovirus encephalitis (Table I). MRI may show
non-specific features of viral encephalitis such as cortical
hyperintensities and cerebral edema. MRI is also useful for diagnosing
alternative etiologies such as Acute disseminated encephalomyelitis, and
antibody-associated encephalopathies.
TABLE I MRI Findings in Viral Encephalitis and Some Mimickers
|
Etiology |
MRI Finding |
|
Herpes simplex encephalitis [63] |
Abnormal signal intensity in medial temporal lobe, cingulate
gyrus, and orbital surface of frontal lobes |
|
Japanese B encephalitis [64, 65] |
Abnormal signal intensity in thalami (87-94%), substantia nigra,
and basal ganglia |
|
EV 71 [66] |
Abnormal signal intensity in the dorsal pons, medulla, midbrain,
and dentate nuclei of the cerebellum; gigh-signal lesions can
also be found in the anterior horn cells of spinal cord in
patients with acute flaccid paralysis |
|
Chandipura virus *[67] |
Normal |
|
Nipah virus [68]* |
Focal subcortical and deep white matter and gray matter lesions;
small hyperintense lesions in the white matter, cortex, pons and
cerebral peduncles have also been seen.
|
|
Varicella [69] |
Multifocal abnormalities in cortex, associated cerebellitis,
vasculitis and vasculopathy |
|
Acute disseminated encephalomyelitis |
Multifocal abnormalities in subcortical white matter;
involvement of thalami, basal ganglia, and brainstem also seen |
|
West Nile virus [70, 71] |
Abnormalities in deep gray matter and brainstem (50%); white
matter lesions mimicking demyelination may also be seen;
meningeal involvement on contrast enhanced images. |
|
*Reports on small number of patients |
Other microbiological investigations: When the
etiology is not clear, other microbiological investigations must be
obtained. These are also required in epidemic situations, where the
etiology has not been established. The local health authorities must be
informed, and a microbiologist should be consulted when taking the
samples. These samples include urine, throat swab, nasopharyngeal
aspirate, serum (acute, and convalescent after 2 weeks), and swab from
vesicles or rash, if present. The duration of time the virus remains in
the CSF may be brief; hence, CSF positivity for some viruses e.g.,
enterovirus is very low. Therefore, it is important to collect and store
these samples. The methods for collection, storage and transport of the
samples are detailed in Table II. Details about the
specific etiologies are given in Table III.
TABLE II Guidelines for Collection, Storage and Transport of Samples
|
Type of sample
|
Guidelines |
|
Blood |
•Collect within 4 days after the onset of illness for isolation
of virus and at least 5 days after the onset of illness for
detection of IgM antibodies.
|
|
•A second, convalescent sample should be collected at least
10-14 days after the first sample for serology.
|
|
•Take clotted blood sample. Separate serum after clot
retraction. |
|
• Serum should be shipped on wet ice within 48 hours or stored
at for a maximum period of 7 days. |
|
•In case a delay is anticipated, sera must be frozen at -20°C
and should be transported to the specified laboratory on frozen
ice packs.
|
|
•Repeated freezing and thawing can have detrimental effects on
the stability of IgM antibodies. |
|
Cerebrospinal fluid |
•Send for cell count, bacteriology, biochemistry and virology
-PCR, serology. |
|
•May be stored at +4°C if delays in processing for virus culture
or viral PCR will be less than 24 hrs. |
|
•If greater delays are likely, CSF should be frozen at -80°C. |
|
Swabs (naso-phary-geal, throat, vesicle) |
•Dacron/ Nylon swabs should be used, and put into virus
transport medium. Swabs may be utilized for a range of virus
cultures and PCR. |
|
Urine |
10-20 mL of urine should be collected into sterile containers
(without preservatives) for mumps virus culture and mumps PCR;
store at -20°C. |
|
Stool |
Stool should be collected for enterovirus culture into clean
containers; store at -20°C. |
|
Brain biopsy |
•Brain specimens should be collected unfixed into a sterile
container. Brain smears can be used for viral antigen detection
by immunofluorescent antibody staining, and for electron
microscopy with negative staining. |
|
•Emulsified brain tissue is suitable for tissue culture and
after proteinase K treatment for PCR. |
|
TABLE III Microbiological Investigations
Available In Acute Encephalitis Syndrome
|
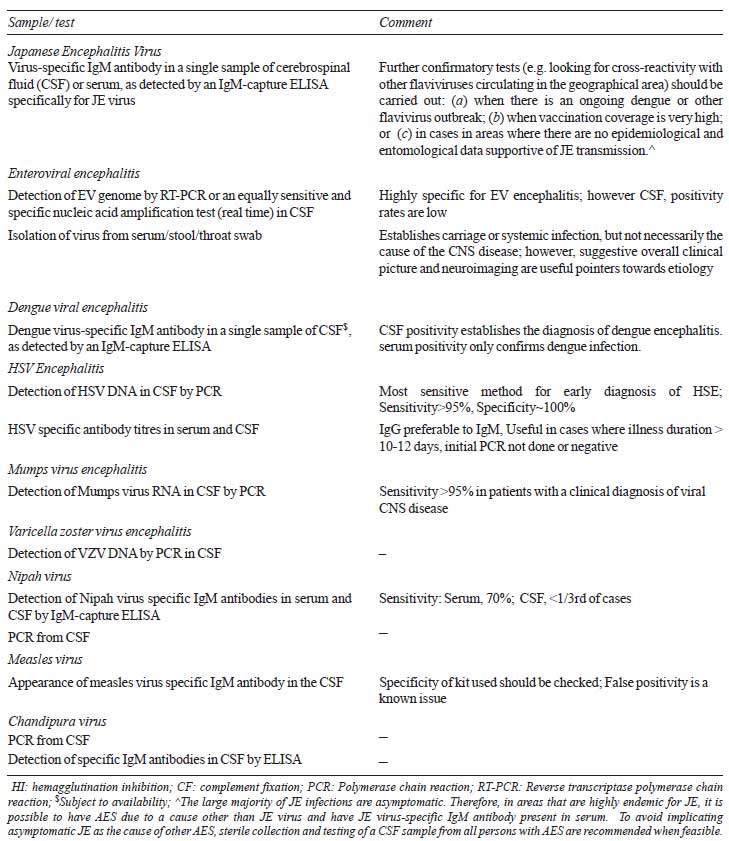 |
Tests for JE, HSV 1 and 2, dengue, poliovirus,
measles, mumps and rubella are available in selected government and
private laboratories. Tests for nipah virus, VZV, EBV, adenovirus and
enterovirus are not easily available. There are commercially available
tests, which test for a panel of viruses (HSV1, 2, VZV, HHV-6, Measles,
Mumps, Rubella, Chandipura, Chikungunya, Nipah, Rabies, Enteroviruses,
Japanese B, Dengue, West Nile virus) and bacteria with 1-2 mL CSF
sample, using DNA hybridization technique and very short turnaround
time. However, these are prohibitively expensive at present. The
sensitivity and specificity of these tests has also not been reported in
published literature. Moreover, the flaviviruses WNV, DENV, and JEV
share some common features, such as transmission via mosquitoes, and
cross-react with each other in serological tests. These cross-reactive
responses could confound the interpretation during serological testing,
including neutralization tests and enzyme-linked immunosorbent assay
(ELISA) [45,46].
In patients having unexplained encephalopathy with
fever and rash, testing for rickettsial infections (Weil- Felix test,
rickettsial serology) must be performed. HIV testing should be performed
in children with unexplained encephalitis. Children with undiagnosed
advanced HIV disease can present with CNS infections from rare causes,
such as cytomegalovirus [1]; and rarely, meningoence-phalitis may be a
presenting feature of primary HIV infection.
Other tests: EEG is not routinely needed as it
usually shows non-specific slowing in viral encephalitis. The presence
of periodic lateralized epileptiform discharges may indicate underlying
herpes simplex encephalitis, but their absence does not rule out the
diagnosis. However, EEG must be performed in children with unexplained
altered sensorium to look for suspected non-convulsive status
epilepticus. EEG may also be helpful in patients with subtle and
doubtful seizures, to guide anti-epileptic drug management.
If the diagnosis is not clear with the above
mentioned tests, then alternative etiologies must be explored. In young
children with unexplained altered sensorium, especially with pre-morbid
developmental delay, investigations for inborn errors of metabolism
(plasma ammonia, blood tandem mass spectroscopy, urine gas
chromatography mass spectroscopy) must be carried out. In older
children, the possibility of autoimmune disorders such as SLE
(anti-nuclear antibodies, anti-ds-DNA antibodies), Hashimoto
encephalopathy (anti-TPO antibodies), and anti-NMDA receptor and
anti-VKGC antibody-mediated encephalitis may be considered. A urine
toxicology screen should be performed. Finally, a brain biopsy may be
needed to look for primary CNS vasculitis or neoplastic processes.
Step 4: Empirical Treatment
Empirical treatment must be started, pending the
results of investigations. A broad spectrum antibiotic such as
ceftriaxone must be given, which can be stopped if no evidence of
bacterial meningitis is forthcoming.
Even though epidemiological data on HSE from India is
lacking, the consensus recommendation of the expert group is that
acyclovir must be started in all cases of sporadic viral encephalitis,
as HSE is a treatable disease. Acyclovir should be stopped if an
alternative diagnosis has been made, or HSV PCR in the CSF is negative
and MRI is normal. However, if the CSF PCR for HSV or MRI have been
performed very early after symptom onset (within 48 hours), these may be
falsely negative. Hence, these studies should be repeated before
stopping acyclovir if the clinical suspicion of HSE continues to be
high. The dose and duration of acyclovir therapy is given in Box
6.
Box 6: Dose and Duration of Acyclovir in Children with Encephalitis^
Dose*
3 mo to 12 y: 500mg/m2 8 hourly
>12 y: 10mg/Kg 8 hourly
Duration
Confirmed cases: 14-21 d intravenous treatment; Minimum 21 d for
those aged 3mo-12y#
Where therapy was started empirically; Stop acyclovir, if an
alternative diagnosis is confirmed, or if HSV PCR in the CSF is
negative on two occasions (24-48 h apart) and MRI imaging does
not suggest HSE. |
|
^Based on reference [50]; *Dose to be reduced in
those with pre-existing renal failure; #CSF-PCR for
HSV may be done at 14-21 days and treatment continued till CSF
is negative. |
Empirical anti-malarial (artemisin-based combination
therapy) must be started if there is a suspicion of cerebral malaria.
This should be stopped if the peripheral smear and rapid diagnostic
tests are negative.
Step 5: Supportive Care
After stabilization of airway, breathing and
circulation, other supportive care measures must be instituted along
with the empirical treatment as mentioned above. Timely and appropriate
supportive care is of paramount importance to reduce the mortality and
morbidity associated with viral encephalitis. Patients with GCS< 8,
having features of raised intracranial pressure, status epilepticus and
shock should ideally be managed in an intensive care unit; however, this
may not always be possible in resource-constrained settings. The
following are the components of supportive care:
(a) Maintenance intravenous fluids:
Fluid therapy should be targeted to maintain euvolemia and
normoglycemia, and to prevent hyponatremia. Children with acute viral
encephalitis should receive fluids at the normal daily requirement.
Increased fluid and fluid boluses may be indicated for dehydration and
hypotension. Isotonic fluids are preferred, and hypotonic fluids (e.g.
0.18% saline in 5% dextrose, Isolyte P) must be avoided, especially in
the presence of raised intracranial pressure. Serum sodium should be
monitored, and abnormalities of serum sodium should be corrected slowly.
If there are features of syndrome of inappropriate secretion of
anti-diuretic hormone, only then fluids should be restricted to
two-thirds of the daily maintenance.
(b) Management of raised intracranial
pressure: Raised intracranial pressure is a common cause of
death in children with viral encephalitis. It is important to recognize
and promptly manage signs of raised ICP. A common mistake in the
emergency departments is to mistake decerebrate posturing for seizures,
and inappropriately treat with anti-epileptic drugs. Intracranial
pressure monitoring is available in very few centers. Therefore,
clinical parameters have to be used to guide the treatment. Attempt
should be made to maintain the cerebral perfusion pressure (CPP), which
is the major factor that affects cerebral blood flow and hence, adequate
oxygenation. CPP depends on the mean arterial pressure and the ICP (CPP=
MAP– ICP). CPP can reduce as a result of reduced MAP or raised ICP or
combination of these two. Therefore, adequate mean arterial pressure
should be maintained.
The following steps are used in the management of
raised intracranial pressure [47]. The patient should undergo intubation
if the GCS is less than 8, or if there is evidence of herniation, or if
the patient has irregular respirations and inability to maintain airway.
If there are signs of impending herniation, then the patient should be
hyperventilated to a target PaCO 2
of 30-35 mm Hg. Mannitol should be given at a dose of initial bolus of
0.25 g/kg, then 0.25 g/kg, q 6 h as per requirement, up to 48 hours.
Hypertonic (3%) saline is preferable to mannitol in the presence of
hypotension, hypovolemia, and renal failure. The dose is 0.1–1 mL/kg/hr
by infusion; the serum sodium should be targeted to a level of 145-155
meq/L [48]. The patient should have adequate sedation and analgesia.
Noxious stimuli should be avoided; nebulized lignocaine should be
administered prior to endotracheal tube suctioning.
(c) Maintain euglycemia: Identify and
treat hypoglycemia with intravenous dextrose (2 mL/kg 10% dextrose, then
glucose infusion rate of 6–8 mg/kg/min). Blood glucose should be
monitored and both hypo- and hyperglycemia should be avoided.
(d) Treatment and prevention of seizures:
If the child is having seizures, or has history of seizures,
anticonvulsant should be administered. A benzodiazepine should be given
(Lorazepam 0.1 mg/kg, diazepam 0.3 mg/kg, or midazolam 0.1 mg/kg)
followed by phenytoin loading (20 mg/kg). Even if there is no history or
clinical evidence of seizures, empirical anticonvulsant therapy may be
considered in children with GCS <8, and features of raised intracranial
pressure [47, 49]. This is because seizures may further raise the
intracranial pressure and thus worsen the outcome.
(e) Other drugs
Corticosteroids: The role of
corticosteroids in the treatment of viral encephalitis is not
established. However, corticosteroids may be considered along with
acyclovir in patients with marked cerebral edema, brain shift or raised
intracranial pressure. Their role remains controversial because steroids
may theoretically increase viral replication [1]. However a
retrospective analysis of 45 adults with HSV encephalitis showed that
lack of administration of corticosteroids was a significant independent
predictor of a poor outcome [50]. Trials of adjunctive corticosteroid
treatment in herpes simplex encephalitis are in progress [51]. Steroids
have not shown to be of benefit in JE [52]. Steroids are indicated in
ADEM, Hashimoto encephalopathy, and autoimmune encephalitis.
Antiviral treatment, i.e., Acyclovir is effective
against encephalitis caused by Varicella Zoster virus. The dosage is
same as that for herpes simplex encephalitis [53]. Pleconaril has been
found to be useful in Enterovirus encephalitis and aseptic meningitis,
but in not in EV 71 encephalitis [42,54]. IVIG has been used in EV 71
encephalitis, but the evidence of clinical benefit is not well
established [42, 55]. Oral ribavirin was not found to be useful in
children with Japanese B encephalitis in a randomized controlled trial
[56]. There is experimental evidence of benefit of minocycline in JE
[57]. Movement disorders such as dystonia may need treatment with
trihexyphenidyl.
(f) Other measures: Acid-base
and electrolyte abnormalities should be corrected. Any concurrent
bacterial infections e.g., pneumonia should be treated with appropriate
antibiotics. The patient should be monitored for changing level of
consciousness, fever, seizures, autonomic nervous system dysfunction,
increased intracranial pressure, and speech and motor disturbances.
Nosocomial infections are important complications during
hospitalization, and must be prevented and treated promptly.
Step 6: Prevention/treatment of complications and
rehabilitation
Nosocomial infections, aspiration pneumonia, and
coagulation disturbances may occur as complications, and should be
detected and treated. Myocarditis and pulmonary edema are important
complications of EV 71 encephalitis. Milrinone has been shown to be of
benefit in these patients. Regular posture change must be done to
prevent the development of bed sores. The patient should be started on
early physiotherapy, to prevent the development of contractures.
Preventive Strategies
Prevention and/or control of AES require a
multi-pronged strategy which should consist of (i) Surveillance
for cases of AES; (ii) Vector control; (iii) Reduction in
man-vector contact; and (iv) Vaccination. Control of vectors and
prevention of man-vector contact are key non-vaccination strategies, but
are beyond the scope of the present communication.
AES Surveillance and Role of Pediatrician in Outbreak
Situations
The purpose of AES surveillance is to estimate
disease burden, to understand disease pattern, and its influence on
mortality and morbidity. Surveillance helps in documenting the burden of
the disease and also helps in proper utilization of scarce resources.
The first and foremost requirement is establishing a proper "case
definition", which can be applied in the field. The same has been
provided here and also by the WHO [2]. Strengthening of surveillance is
urgently needed throughout the country, more so in endemic states where
frequent outbreaks are reported. Sentinel site hospitals should be
identified for disease surveillance and case management, both in endemic
and non-endemic areas. Mechanisms should be developed for AES reporting
both by institutions and individual practitioners. It could even be a
web-based system, as is being done for infectious diseases surveillance
by IAP through IDsurv (http://www.idsurv.org/).
In any AES outbreak, pediatricians will see affected
patients. They should be aware of what information and samples they
should collect, and whom to inform. All pediatricians need to be aware
of the case definition of AES. They should be aware of the common viral
etiologies in their area, and should be alert if there is a clustering
of cases. The cases should be notified to the District Surveillance
Unit. All cases of AES should be notified to the local health
authorities (the IDSP District surveillance unit). The concerned officer
may be informed by telephone, fax, or e-mail. The requisite forms are
available on the IDSP Portal (www.idsp.nic.in). The minimum data to be
collected has been published (58). The samples that need to be
collected, their timing and methods have already been detailed (Table
II).
Immunization
Human vaccination is the only effective, long-term,
cost-effective measure against AES. At-risk population should receive a
safe and efficacious vaccine as part of the national immunization
program. Although vaccines are under development against many viral
agents responsible for AES in children, but primarily it is JE against
which vaccines are available for routine use. Vaccines are currently
under development against Dengue, Enteroviruse 71, and other
flaviviruses like West Nile virus.
JE vaccination
The most effective immunization strategy in JE
endemic settings is a one-time campaign in the primary target
population, as defined by local epidemiological data, followed by
incorporation of the JE vaccine in to the routine immunization program
[59]. This approach has a greater public health impact than either
strategy separately.
The JE vaccines include; a mouse brain derived
inactivated vaccines (high incidence of sometime fatal complications,
currently not available in India); Cell culture-derived, inactivated JE
vaccine based on the Beijing P-3 strain (available only in China); and
Cell culture-derived, live attenuated vaccine based on the SA14-14-2
strain [60]. Some newer JE vaccines are on the horizon (Chimeric vaccine
-IMOJEV by Sanofi Pasteur, Inactivated SA-14-14-2 vaccine (IC51) -IXIARO
by Intercel, Inactivated vero-cell derived JE vaccine-Beijing-1 JE
strain by Biken and Kaketsuken) [61], but would not be discussed further
here. The IAP Guidelines on Immunization provide recommendations on JE
vaccination in India [62].
Cell culture-derived, live attenuated vaccine:
Currently, this is the only JE vaccine available in India. This vaccine
is based on the genetically stable, neuro-attenuated SA 14-14-2 strain
of the JE virus, which elicits broad immunity against heterologous JE
viruses. Reversion to neurovirulence is considered highly unlikely. The
price per dose of the vaccine is comparable to the EPI measles vaccine.
0.5 mL dose is to be administered subcutaneously to children at eight
months of age and a second opportunity again at two years. In some
areas, a booster dose is given at seven years. It should not be used as
an "outbreak response vaccine". It can also be offered to all
susceptible children up to 15 yrs, and should be administered as a
catch-up vaccination [59]. The vaccine should be stored and shipped at
8ºC, protected from sunlight. After a single dose, antibody responses
are produced in 85 to 100% of non-immune 1- to 12-year-old children
[61].
In India, one dose of SA-14-14-2 imported from China
is being used in many states since 2006 [63], and the number of
districts covered under the vaccine have been increased recently.
Children between the age group of 1 to 15 years were vaccinated with a
single dose of SA14-14-2 vaccine, with a coverage >80% [63]. The
efficacy of a single dose of this vaccine was reported to be 94.5% (95%
CI, 81.5 to 98.9) [64]. Preliminary results of recent case control study
carried out by ICMR on impact of JE vaccine shows an unadjusted
protective effect of 62.5% in those with any report of vaccination [65].
Conclusions
Consensus guidelines on evaluation and management of
acute viral encephalitis in Indian children are provided. Early
stabilization and institution of non-specific supportive measures is the
cornerstone of management. Investigations are aimed at recognition of
etiological agent for specific therapeutic and control measures.
Reporting and appropriate workup of all cases would strengthen the AES
surveillance and go a long way in reducing the morbidity and mortality
due to this disorder.
|
Annexure I
List of Participants (in alphabetical order)
Chairperson: Prof T Jacob John, Vellore;
Co-Chair: Dr Rohit Agarwal,Mumbai, President IAP 2012
Prof S. Aneja, LH Medical College, Delhi
(Convener);Dr Milind M Gore, NIV (Gorakhpur unit); Dr S. Gulati, AIIMS,
Delhi; Prof Amita Jain, CSSMU, Lucknow; Prof V Kalra, Indraprastha
Apollo Hospital, Delhi; Prof R Kumar, CSSMU, Lucknow; Prof KP Kushwaha,
BRD Medical College, Gorakhpur; Prof S Mahadevan, JIPMER, Puducherry; Dr
D. Mishra, MA Medical College, Delhi (Co-Convener); Dr Veena Mittal,
NCDC, Delhi; Dr S. Sharma, LH Medical College, Delhi; Prof P Singhi,
PGIMER, Chandigarh; Dr Vipin M Vashistha (Convener, IAPCOI), Bijnor, UP.
Prof V Ravi (NIMHANS, Bangalore) and Dr Rakesh Lodha (AIIMS, Delhi) were
invited but could not attend.
|
| |
Acknowledgements: The infrastructure and
administrative support provided by the Organizing committee of PEDICON
2012, especially by Dr. MP Jain, Organizing Secretary, is gratefully
acknowledged.
Disclaimer: These clinical guidelines have
been developed by expert members of the IAP and are intended to provide
an overview of currently recommended treatment strategies for suspected
viral encephalitis. The usage and application of these clinical
guidelines will take place at the sole discretion of treating
clinicians, who retain professional responsibility for their actions and
treatment decisions.
Contributors: The list of participants in
the Expert group meeting is provided in Annexure 1. All
the members of the writing group made equal contribution to the
literature search and manuscript preparation. Prof. S. Aneja would be
the guarantor for the manuscript.
Competing interests: None stated.
Funding: PEDICON 2012 Organizing Committee
(Indian Academy of Pediatrics).
References
1. Kneen R, Michael BD, Menson E, Mehta B, Easton A,
Hemingway C, et al. Management of suspected viral encephalitis in
children - Association of British Neurologists and British Pediatric
Allergy Immunology and Infection Group National Guidelines. J
Infect. 2012;64:449-77.
2. World Health Oraganisation. Acute Encephalitis
Syndrome. Japanese encephalitis surveillance standards. January 2006.
From WHO-recommended standards for surveillance of selected
vaccine-preventable diseases. WHO/V&B/03.01. Available from: http://www.
who. int/vaccines-documents/DocsPDF06/843.pdf. Accessed on 8 August,
2012.
3. Hollidge BS, Gonzalez-Scarano F, Soldan SS.
Arboviral encephalitides: transmission, emergence, and pathogenesis. J
Neuroimmune Pharmacol.2010; 5:428-42.
4. Sapkal GN, Bondre VP, Fulmali PV, Patil P,
Gopalkrishna V, Dadhania V, et al. Enteroviruses in patients with
acute encephalitis, Uttar Pradesh, India. Emerg Infect Dis.2009;
15:295-8.
5. Karmarkar SA, Aneja S, Khare S, Saini A, Seth A,
Chauhan BK. A study of acute febrile encephalopathy with special
reference to viral etiology. Indian J Pediatr. 2008; 75:801-5.
6. Rao BL, Basu A, Wairagkar NS, Gore MM, Arankalle
VA, Thakare JP, et al. A large outbreak of acute encephalitis
with high fatality rate in children in Andhra Pradesh, India, in 2003,
associated with Chandipura virus. Lancet.2004; 364:869-74.
7. Chadha MS, Arankalle VA, Jadi RS, Joshi MV,
Thakare JP, Mahadev PV, et al. An outbreak of Chandipura virus
encephalitis in the eastern districts of Gujarat state, India. Am J Trop
Med Hyg.2005; 73:566-70.
8. Harit AK, Ichhpujani RL, Gupta S, Gill KS, Lal S,
Ganguly NK, et al. Nipah/Hendra virus outbreak in Siliguri, West
Bengal, India in 2001. Indian J Med Res. 2006; 123:553-60.
9. Kalantri SP, Joshi R, Riley LW. Chikungunya
epidemic: an Indian perspective. Natl Med J India. 2006; 19:315-22.
10. Kumar R, Tripathi S, Tambe JJ, Arora V,
Srivastava A, Nag VL. Dengue encephalopathy in children in Northern
India: clinical features and comparison with non dengue. J Neurol Sci.
2008; 269:41-8.
11. Steiner I. Herpes simplex virus encephalitis: new
infection or reactivation? Curr Opin Neurol. 2011; 24:268-74.
12. Granerod J, Ambrose HE, Davies NW, Clewley JP,
Walsh AL, Morgan D, et al. Causes of encephalitis and differences
in their clinical presentations in England: a multicentre,
population-based prospective study. Lancet Infect Dis. 2010; 10:835-44.
13. Wairagkar NS, Shaikh NJ, Ratho RK, Ghosh D,
Mahajan RC, Singhi S, et al. Isolation of measles virus from
cerebrospinal fluid of children with acute encephalopathy without rash.
Indian Pediatr.2001; 38:589-95.
14. John TJ. Encephalopathy without rash, caused by
measles virus? More evidence is needed. Indian Pediatr. 2003; 40:589-93.
15. Kumar S. Inadequate research facilities fail to
tackle mystery disease. BMJ. 2003; 326:12.
16. Tyler KL. Emerging viral infections of the
central nervous system: part 2. Arch Neurol. 2009; 66:1065-74.
17. Tyler KL. Emerging viral infections of the
central nervous system: part 1. Arch Neurol. 2009; 66:939-48.
18. Benjamin LA, Lewthwaite P, Vasanthapuram R, Zhao
G, Sharp C, Simmonds P, et al. Human parvovirus 4 as potential
cause of encephalitis in children, India. Emerg Infect Dis. 2011;
17:1484-7.
19. Khan SA, Dutta P, Khan AM, Chowdhury P, Borah J,
Doloi P, et al. West Nile virus infection, Assam, India. Emerg
Infect Dis. 2011; 17:947-8.
20. Thakare JP, Rao TL, Padbidri VS. Prevalence of
West Nile virus infection in India. Southeast Asian J Trop Med Public
Health. 2002; 33:801-5.
21. Bondre VP, Sapkal GN, Yergolkar PN, Fulmali PV,
Sankararaman V, Ayachit VM, et al. Genetic characterization of
Bagaza virus (BAGV) isolated in India and evidence of anti-BAGV
antibodies in sera collected from encephalitis patients. J Gen Virol.
2009;90:2644-9.
22. Kumar A, Shukla D, Kumar R, Idris MZ, Misra UK,
Dhole TN. An epidemic of encephalitis associated with human enterovirus
B in Uttar Pradesh, India, 2008. J Clin Virol. 2011; 51:142-5.
23. Glaser CA, Honarmand S, Anderson LJ, Schnurr DP,
Forghani B, Cossen CK, et al. Beyond viruses: clinical profiles
and etiologies associated with encephalitis. Clin Infect Dis. 2006;
43:1565-77.
24. Vashishtha VM, Kumar A, John TJ, Nayak NC.
Cassia occidentalis poisoning causes fatal coma in children in
western Uttar Pradesh. Indian Pediatr.2007; 44:522-5.
25. Sriramachari S. Heat hyperpyrexia: time to act.
Indian J Med Res.2004; 119:vii-x.
26. Ghosh D, Dhadwal D, Aggarwal A, Mitra S, Garg SK,
Kumar R, et al. Investigation of an epidemic of Reye’s syndrome
in northern region of India. Indian Pediatr.1999;36:1097-106.
27. John TJ, Date A, Patoria NK. Acute encephalopathy
in children in Nagpur: similarity to Reye’s syndrome. Indian J Pediatr.
1983; 50:129-32.
28. John TJ. Outbreak of killer brain disease in
children: mystery or missed diagnosis? Indian Pediatr. 2003;40:863-9.
29. Japanese Encephalitis Clinical Care Guidelines.
PATH, November, 2006. Available from:
www.path.org/vaccineresources/files JE_clinical_care_guidelines_ PATH.
pdf. Accessed on 3 August, 2012.
30. Ministry of Health and Family Welfare, Government
of India. Facility-based IMNCI (F-IMNCI) Participants Manual. Government
of India, New Delhi, 2009. Available from:www.unicef.org/india/FBC_Participants_
Manual.pdf. Accessed on 3 August, 2012.
31. Davis LE, Tyler KL. Molecular diagnosis of CNS
viral infections. J Neurol Neurosurg Psychiatry. 2005; 76:10.
32. Sharma S, Kochar GS, Sankhyan N, Gulati S.
Approach to the child with coma. Indian J Pediatr. 2010; 77:1279-87.
33. Wang GF, Li W, Li K. Acute encephalopathy and
encephalitis caused by influenza virus infection. Curr Opin Neurol.
2001; 23:305-11.
34. John TJ, Maiya PP, Jadhav M, Christopher S,
Mukundan P. Mumps virus meningitis and encephalitis without parotitis.
Indian J Med Res. 1978; 68:883-6.
35. Johnstone JA, Ross CA, Dunn M. Meningitis and
encephalitis associated with mumps infection. A 10-year survey. Arch Dis
Child. 1972; 47:647-51.
36. Elbers JM, Bitnun A, Richardson SE, Ford-Jones
EL, Tellier R, Wald RM, et al. A 12-year prospective study of
childhood herpes simplex encephalitis: is there a broader spectrum of
disease? Pediatrics. 2007;119:e399-407.
37. Kirkham FJ, Newton CR, Whitehouse W. Paediatric
coma scales. Dev Med Child Neurol. 2008; 50:267-74.
38. Stevens RD, Bhardwaj A. Approach to the comatose
patient. Crit Care Med. 2006; 34:31-41.
39. Steer AC, Starr M, Kornberg AJ. Bickerstaff
brainstem encephalitis associated with Mycoplasma pneumoniae
infection. J Child Neurol. 2006; 21:533-4.
40. Taylor DA. Impairment of consciousness and coma.
Philadelphia: Elsevier, 2006.
41. Brazis PW, Biller J. Coma. Philadelphia:
Lippincott Williams and Wilkins, 2001.
42. Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ,
Solomon T. Clinical features, diagnosis, and management of enterovirus
71. Lancet Neurol. 2010; 9:1097-105.
43. Kumar R, Tripathi P, Singh S, Bannerji G.
Clinical features in children hospitalized during the 2005 epidemic of
Japanese encephalitis in Uttar Pradesh, India. Clin Infect Dis. 2006;
43:123-31.
44. Schemann JF, Doumbo O, Malvy D, Traore L, Kone A,
Sidibe T, et al. Ocular lesions associated with malaria in
children in Mali. Am J Trop Med Hyg. 2002; 67:61-3.
45. Kuno G. Serodiagnosis of flaviviral infections
and vaccinations in humans. Adv Virus Res. 2003; 61:3-65.
46. Hua R, Chen N, Qin C, Deng Y, Ge J, Wang X, et
al. Identification and characterization of a virus-specific
continuous B-cell epitope on the PrM/M protein of Japanese encephalitis
virus: potential application in the detection of antibodies to
distinguish Japanese encephalitis virus infection from West Nile virus
and Dengue virus infections. Virology J. 2010; 7:249.
47. Sankhyan N, Vykunta Raju KN, Sharma S, Gulati S.
Management of raised intracranial pressure. Indian J Pediatr. 2010;
77:1409-16.
48. Suarez JI. Hypertonic saline for cerebral edema
and elevated intracranial pressure. Cleve Clin J Med. 2004; 71 Suppl
1:S9-13.
49. Rabinstein AA. Treatment of cerebral edema.
Neurologist. 2006; 12:59-73.
50. Kamei S, Sekizawa T, Shiota H, Mizutani T,
Itoyama Y, Takasu T, et al. Evaluation of combination therapy
using aciclovir and corticosteroid in adult patients with herpes simplex
virus encephalitis. J Neurol Neurosurg Psychiatry.2005; 76:1544-9.
51. Martinez-Torres F, Menon S, Pritsch M, Victor N,
Jenetzky E, Jensen K, et al. Protocol for German trial of
acyclovir and corticosteroids in Herpes-simplex-virus-encephalitis
(GACHE): a multicenter, multinational, randomized, double-blind,
placebo-controlled German, Austrian and Dutch trial (ISRCTN45122933).
BMC Neurol. 2008; 8:40.
52. Hoke CH Jr., Vaughn DW, Nisalak A, Intralawan P,
Poolsuppasit S, Jongsawas V, et al. Effect of high-dose
dexamethasone on the outcome of acute encephalitis due to Japanese
encephalitis virus. J Infect Dis. 1992; 165:631-7.
53. Steiner I, Budka H, Chaudhuri A, Koskiniemi M,
Sainio K, Salonen O, et al. Viral meningoencephalitis: a review
of diagnostic methods and guidelines for management. Eur J Neurol. 2010;
17:999-e957.
54. Rotbart HA, Webster AD. Treatment of potentially
life-threatening enterovirus infections with pleconaril. Clin Infect
Dis. 2001; 32:228-35.
55. Wang SM, Lei HY, Huang MC, Su LY, Lin HC, Yu CK,
et al. Modulation of cytokine production by intravenous
immunoglobulin in patients with enterovirus 71-associated brainstem
encephalitis. J Clin Virol. 2006; 37:47-52.
56. Kumar R, Tripathi P, Baranwal M, Singh S,
Tripathi S, Banerjee G. Randomized, controlled trial of oral ribavirin
for Japanese encephalitis in children in Uttar Pradesh, India. Clin
Infect Dis. 2009;48:400-6.
57. Dutta K, Kumawat KL, Nazmi A, Mishra MK, Basu A.
Minocycline differentially modulates viral infection and persistence in
an experimental model of Japanese encephalitis. J Neuroimmune Pharmacol.
2010; 5:553-65.
58. George K. Investigating outbreaks of uncertain
aetiologies. Indian J Med Res. 2007;125:505-7.
59. Japanese encephalitis vaccines. Wkly Epidemiol
Rec. 2006;81:331-40.
60. Fischer M, Lindsey N, Staples JE, Hills S.
Japanese encephalitis vaccines: recommendations of the Advisory
Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;
59:1-27.
61. Halstead SB, Thomas SJ. New Japanese encephalitis
vaccines: alternatives to production in mouse brain. Expert Rev
Vaccines. 2011;10:355-64.
62. Indian Academy of Pediatrics. IAP Guidebook on
Immunization, IAP, Mumbai, 2009. Available from:
http://www.indg.in/health/child-health/IAP%20GUIDE%
20BOOK%20ON%20IMMUNIZATION%202009-2011.pdf. Accessed on 8 August, 2012.
63. Government of India. Operational Guide Japanese
Encephalitis Vaccination in India. Immunization Division Department of
Family Welfare, Ministry of Health and Family Welfare, Government of
India; September 2010. Available from http://health.bih.nic.in/
Docs/Guidelines-Japanese-Encephalitis.pdf. Accessed on 11 October, 2012.
64. Kumar R, Tripathi P, Rizvi A. Effectiveness of
one dose of SA 14-14-2 vaccine against Japanese encephalitis. N Engl J
Med. 2009; 360:1465-6.
65. Indian Council of Medical Research. Minutes of
the meeting of the Core Committee on Vaccines. Available from:http://www.icmr.nic.in/minutesMinutes%20
Core%20Committee%20on%20Vaccines. pdf. Accessed on March 28, 2012.
|
|
|
 |
|

