|
|
|
Indian Pediatr 2010;47: 945-953 |
 |
Gallstone Disease in Children |
|
Ujjal Poddar
Correspondence to: Dr Ujjal Poddar, Department of
Pediatric Gastroenterology, Sanjay Gandhi Post Graduate
Institute of Medical Sciences, Raebareli Road, Lucknow 226 014, Uttar
Pradesh, India.
Email: [email protected]
|
Context: Little is known about the epidemiology of cholelithiasis
in children. Cholelithiasis and choledocholithiasis were considered to be
uncommon in infants and children but have been increasingly diagnosed in
recent years due to wide- spread use of ultrasonography. However, there is
not much of information from India and no consensus among Indian
pediatricians and pediatric surgeons regarding management of gallstones in
children. Hence, the purpose of this review is to increase awareness about
the management of gallstones in children. Methods: Extensive
electronic (PubMed) literature search was made for this purpose and
literature (original articles, clinical trials, case series, review
articles) related to gallstones in children were reviewed. Conclusions:
The etiologies of cholelithiasis are hemolytic (20%-30%), other known
etiology (40%-50%) such as total parenteral nutrition, ileal disease,
congenital biliary diseases, and idiopathic (30-40 %). Spontaneous
resolution of gallstones is frequent in infants and hence a period of
observation is recommended even for choledocholithiasis. Children with
gallstones can present with typical biliary symptoms (50%), nonspecific
symptoms (25%), be asymptomatic (20%) or complicated (5%-10%).
Cholecystectomy is useful in children with typical biliary symptoms but is
not recommended in those with non-specific symptoms. Prophylactic
cholecystectomy is recommended in children with hemolytic disorders.
Key words: Choledocholithiasis, Cholelithiasis, Outcome.
|
|
Cholelithiasis
or gallstones are quite common in adults. The prevalence of gallstones
among adult population in the West is 10% to 20% (1,2) and this figure in
India is 3% to 6%(3,4). Interestingly the prevalence of gallstones is
seven times more frequent in north India than in south India(5) and the
composition of gallstones is also different in different parts India. In
north and eastern India, gallstones are predominantly cholesterol stones
and mixed stones; on the other hand, in south India, pigment stones are
predominant(6,7). The natural history of gallstones in adults has shown
that the majority (more than 80%) are incidentally detected asymptomatic
gallstones(8) and the majority of them (>80%) remains asymptomatic on long
term follow up; even if they develop complications (like pancreatitis,
cholecystitis, choledocholithiasis), they are usually preceded by biliary
colic(1,9). Hence, in adults, prophylactic cholecystectomy is not recommended
for asymptomatic gallstones. However, the picture is not so clear in
children.
Gallstones were considered to be uncommon in infants
and children but have been increasingly diagnosed in the recent years,
mainly due to wide spread use of ultrasonography. There is not much
information about cholelithiasis in children from India and there is no
consensus among Indian pediatricians and pediatric surgeons regarding the
management of gallstones in children. Hence, the purpose of this review is
to increase awareness about the management of gallstones in children.
Methods
A detailed electronic (PubMed) literature search was
made for this purpose and English language literature (original articles,
clinical trials, case series, case report, letter, meta-analysis, practice
guideline, randomized controlled trial) related to gallstones in children
(key words used: gallstones, children, ceftriaxone, fetal gallstones) were
reviewed. The period of search was from 1965 to December 2009. During this
period a total of 372 articles were published of which relevant 61
articles were included in this review.
Epidemiology
Little is known about the epidemiology of
choleli-thiasis in children. Cholelithiasis and choledo-cholithiasis have
been increasingly diagnosed in recent years in children. This phenomenon
may be attributed to better medical imaging (especially ultrasonography)
and its usage in investigating children with unexplained abdominal pain
and/or a genuine increase in the incidence of cholelithiasis due to
increasing use of total parenteral nutrition, frusemide and phototherapy
in the infants(10). The exact prevalence of gallstones in children is not
known. Studies from Europe have shown an overall prevalence of gallstone
disease of 0.13% to 0.2% in children(11,12).
In Japan, the prevalence of gallstone disease is reported to be less than
0.13% of children(13). The only report from India by Ganesh, et al.
has shown a prevalence of 0.3% in a hospital based observation among
13,675 children(14). However, the prevalence of gallstones among obese
children and adolescents was shown to be quite high (2% of 493 children)
in a recent study(15). Studies on cholelithiasis in children have shown a
bimodal distribution, with a small peak in infancy and a steadily rising
incidence from early adolescence onwards(11,16). Boys and girls are
equally affected in early childhood, but as in adults, a clear female
preponderance emerges during adolescence.
A unique subset is chronic hemolytic disease. In this
condition, cholelithiasis is usually not seen before the age of five and
thereafter, the incidence increases progressively with age. In sickle-cell
disease, the prevalence of pigment gallstones was reported to be 10% to
15% in children under 10 years of age, it increased to 40% in those aged
10-18 years, and 50% in adults(17-19). The prevalence of gallstones in
hereditary spherocytosis was 10% to 20% and in adult series it was
40%(20,21). In thalassemia, the reported figure is low (10% to
15%)(22,23). With longer survival of thalassemia patients, higher
prevalence of gallstones (50%) has been reported(24). The highest
prevalence of gallstones have been reported in thalassemics with Gilbert’s
syndrome genotype(24,25). However, in a study on 64 patients with median
age of 10 (range, 5 to 20) years with thalassemia major from Chandigarh,
none had gallstones(26).
Pathogenesis
Gallstones are either cholesterol gallstones (pure and
mixed) or pigment stones (black or brown). Cholesterol supersaturation of
bile with stasis predisposes to cholesterol gallstone formation. Mixed
cholesterol gallstones are the commonest stones in adults and in
adolescent girls. However, pigment stones are more common in children.
Black pigment stones are formed due to supersaturation of bile with
calcium bilirubinate and are seen in hemolytic disorders and in
association with total parenteral nutrition. Brown pigment stones are
associated with infection and biliary stasis and form more often in the
bile ducts than in the gallbladder(16).
Biliary sludge is composed of mucin, calcium
bilirubinate and cholesterol crystals. It is commonly associated with
prolonged fasting, total parenteral nutrition, pregnancy, sickle-cell
disease, treatment with ceftriaxone or octreotide(27). The natural history
of biliary sludge is variable; it may resolve spontaneously or may
progress to gallstone develop-ment. Persistent sludge may give rise to
biliary complications (such as obstruction or infection).
Etiologically cholelithiasis (Table I)(11,16)
in children can be divided into three groups; hemolytic, other known
etiology, and idiopathic. Almost 20% to 30% of all gallstones in children
are due to hemolytic diseases such as sickle-cell disease, hereditary
spherocytosis and thalassemia. In around 40% to 50% of cases, gallstones
are due to another known etiology such as total parenteral nutrition,
prolonged fasting, ileal disease or ileal resection, frusemide therapy,
congenital biliary diseases such as choledochal cyst, chronic liver
disease and progressive familial intrahepatic cholestasis (PFIC). Around
30% to 40% of cases are idiopathic. As in adults, gallstones in adolescent
girls are more often idiopathic(16).
TABLE I
Etiology of Gallstones in Children(11,16)
|
Type of gallstones |
Proportion of all gallstones
in children |
Etiology |
|
Hemolytic |
20%-30% |
Sickle-cell disease, hereditary spherocytosis, thalassemia major |
|
Non-hemolytic |
40%-50% |
TPN, prolonged fasting, ileal disease (like Crohn’s disease) or
resection, prematurity, frusemide therapy, cardiopulmonary bypass,
congenital biliary malformations, PFIC, chronic liver disease,
cystic fibrosis, OCP, teenage pregnancy |
|
Idiopathic |
30-40% |
No predisposing factor |
|
TPN: total parenteral nutrition, PFIC:
progressive familial intrahepatic cholestasis, OCP: oral
contraceptive pills |
Total parenteral nutrition and cholelithiasis
Total parenteral nutrition (TPN) impairs entero-hepatic
circulation and cholecystokinin induced gallbladder contraction resulting
in biliary stasis, sludge and stones(28). The longer the duration of TPN
therapy, the higher the risk of developing cholelithiasis(29). The risk of
developing gallstones in children on prolonged TPN therapy is increased if
there is concomitant ileal resection or disease. In a study of 21 children
on prolonged TPN (more than 3 months), Roslyn, et al. have shown
that 43% of children developed gallstones but this figure was 64% in
children with ileal resection or disease(29). Sludge develops more rapidly
in neonates than in adults after a mean time of TPN infusion in neonates
of 10 days, as compared with more than 6 weeks in adults. In a prospective
study of 41 neonates, Matos, et al. have shown that gallbladder
sludge appeared in 18 (44%) of those infants who had received TPN infusion
for a mean period of 10 days(30). In 12 infants, the sludge cleared within
one week of resuming enteral feeding but two of the remaining infants went
on to develop asymptomatic gallstones. Spontaneous resolution occurred in
one of these two infants by 6 months while the calculi persisted in the
other baby.
Ceftriaxone-associated biliary pseudolithiasis
Ceftriaxone, a third-generation cephalosporin, is a
popular drug among pediatricians as it has a broad spectrum of
antimicrobial activity and good CSF penetration. Biliary sludge or biliary
lithiasis has been reported as a potential complication of ceftri-axone
treatment since 1986(31). Since ceftriaxone induced biliary lithiasis is
reversible and disappears on discontinuation of therapy it is called ‘pseudoli-thiasis’.
In a patient with normal renal function, 60% of the drug is excreted
unchanged into the urine and 40% is excreted into the bile(32).
Ceftriaxone is an anion, can concentrate in bile 20 to 150 times more than
in serum and readily forms an insoluble salt with calcium (calcium-ceftriaxone)
that precipitates in gallbladder(33). The risk factors for ceftriaxone
pseudolithiasis are hypercalcemia, renal failure (leads to increase
biliary concentration), high dose (>2g or >200mg/kg/day), long-term
treatment and gallbladder stasis(34).
The incidence of ceftriaxone induced pseudolithiasis is
15% to 46% in various prospective studies(38-42). Usually pseudoliths
appear after 6 (range, 3 to 22) days of therapy and disappear after 15
(range, 2-63) days of discontinuation of therapy(35,36). Most cases of
ceftriaxone induced pseudolithiasis are asymptomatic and detected on
sonography but rarely (0-19% of cases)(36), they can produce symptoms like
pain abdomen, nausea, vomiting and biliary obstruction. In symptomatic
cases, discontinuation of drug is recommended. However, cessation of drug
therapy is unnecessary in incidentally detected asymptomatic cases of
ceftri-axone induce pseudolithiasis.
Low phospholipid associated cholelithiasis (LPAC)
Gallstones in the adolescent age group with a strong
family history of gallstones under the age of 40 years, intrahepatic
cholestasis of pregnancy or a cholestatic reaction to oral contraception
should raise the suspicion of a rare condition called low phospholipid
associated cholelithiasis (LPAC)(37). The underlying cause is a mutation
in MDR3, a gene which encodes the ABCB4 transporter. This protein is
responsible for the translocation of phosphatidyl-choline across the
canalicular membrane of hepato-cytes which then solubilises cholesterol in
bile. In the absence of phosphatidylcholine, bile becomes supersaturated
with cholesterol and predisposes to gallstone formation. This condition is
more frequent in females than in males (3:1). Apart from a family history
of cholesterol gallstones amongst first degree relatives, intrahepatic
hyperechoic foci (cholesterol crystal deposits in intrahepatic bile ducts)
are a characteristic sign of the LPAC syndrome. The typical biliary
symptoms experienced by these patients are probably due to cholesterol
crystal deposits and bile duct inflammation as symptoms recur in more than
half of the cases after cholecystectomy. Ursodeoxycholic acid (UDCA)
appears to relieve biliary symptoms long before the dissolution of
intrahepatic stones(38).
Clinical presentation
Table II summarizes the clinical presentations
at different age of presentation.
TABLE II
Clinical Presentations of Gallstones in Children(46,47)
| Age group |
Proportion of all gallstones in children |
Clinical presentations |
| Infants (< 2yrs) |
10% |
1. Symptomatic: Cholestatic jaundice, transient
acholic stools, abdominal pain, and sepsis. 2. Asymptomatic:
incidental detection. |
|
Children (2-14 yrs) |
40% |
1. Typical biliary symptoms (40%-50%): right upper quadrant or
epigastric pain with or without nausea, vomiting and fat
intolerance. |
| |
|
2. Non-specific abdominal pain (20%-30%). |
| |
|
3. Acute abdomen (5%-10%): due to acute cholecystitis, pancreatitis
or cholangitis. |
| |
|
4. Asymptomatic (20%). |
| Adolescents(14-18 yrs) |
50% |
Same as in children but right upper quadrant pain
and fatty food intolerance are more common in adolescents than in
children. |
Fetal Cholelithiasis
The prenatal diagnosis of fetal gallstones has been
reported since 1983(39) but is a rare finding and little is known about
the natural history and clinical significance. The most cases of fetal
cholelithiasis reported in the literature are detected in the third
trimester of pregnancy with no apparent sex predilection and all were
identified as incidental findings. The echogenic material detected in
fetal gallbladder is usually sludge as in most reported cases there was
lack of acoustic shadowing. The prognosis of fetal gallstones is very good
as complete spontaneous resolution has been documented in the majority of
cases between 1 and 12 months after birth and those that persist are
rarely symptomatic(40). There are several hypotheses put forward to
explain the formation of fetal gallstones but none are conclusive. Brown,
et al. have suggested that increased level of estrogen might
predispose to the formation of stones by increasing the secretion of
cholesterol and reducing the synthesis of bile acids(41). Other possible
predisposing factors are: use of narcotics in pregnant women, hemolytic
anemia, blood group incompatibility, structural abnormalities like
choledochal cyst, pregnancy induced cholestasis, etc.(42,43).
Cholelithiasis in infancy
Cholelithiasis is uncommon in infants but increasing
numbers are reported in recent years mainly due to increasing use of
ultrasonography (US). Gallstones in infancy are usually asymptomatic but
occasionally can present with cholestatic jaundice, transient acholic
stools, sepsis and abdominal pain. In symptomatic infants, gallstones are
more often associated with stones in the common bile duct (CBD) than
stones in the gallbladder alone(44,45). In a series of 13 cases of
cholelithiasis in infants, St-Vil, et al. have reported that 11
were asymptomatic and detected by US done for unrelated problems(44). In
another series of 40 cases of cholelithiasis in infancy, Debray, et al.
have shown that 6 had isolated gallstones and were asymptomatic whereas 34
had bile duct obstruction and were symptomatic(45).
Cholelithiasis in children
Children with gallstones can present with acute
abdominal pain due to cholecystitis, cholangitis or pancreatitis. However,
an acute presentation is uncommon (5% to 10% of cases only). Most
commonly, children with cholelithiasis present with typical right upper
quadrant pain (50%) or non-specific abdominal symptoms (25%) including
poorly localized abdominal pain and nausea. Around 20% of cases are
asymptomatic (incidentally detected stones)(11,46,47). Gallstones in
children are more often (60%) symptomatic than in adults (20%)(46). The
type of symptoms depend on the age of presentation, older children (6
years or more) often localize pain in the right upper quadrant whereas
younger children (5 years or less) tend to present with nonspecific
symptoms(11). Fatty food intolerance, a typical symptom of gallstone
disease in adults, tends to be reported by older children(47).
Cholelithiasis in adolescents
In this age group, the symptoms are similar to those
reported in adults. Fatty food intolerance, biliary colic and acute or
chronic cholecystitis are usual presenting features of symptomatic
patients(47).
Diagnosis
The universally used and the most accurate diagnostic
test in detecting the presence of gallstones is ultrasonography.
Gallstones are usually mobile, single or multiple and characteristically
cast an acoustic shadow. Biliary sludge though appearing echogenic on
ultrasound, does not cast an acoustic shadow. A stone, as small as 1.5 mm,
can be detected by ultrasonography. The sensitivity and specificity of
ultrasonography exceeds 95% for gallbladder cholelithiasis, but this
figure is only 50%-75% for choledocholithiasis. In children 20% to 50%
gallstones are radiopaque(48).
Cholescintigraphy, with technetium 99 m
labeled diisopropyl iminodiacetic acid (DISIDA), is the most accurate
method of diagnosing acute cholecystitis. Nonvisulization of the
gallbladder in an otherwise patent biliary system suggests acute
cholecystitis. Magnetic resonance cholangio-pancreatography (MRCP) is
being used increasingly to investigate complicated gallstone disease.
Endoscopic retrograde cholangiopancreatography (ERCP) offers the
additional advantage of therapeutic intervention in common bile duct
stones(49,50).
Management
Management of gallstones depends on the symptoms and
the age of the patient. Symptomatic gallstones need cholecystectomy and
same is true for complicated gallstones but there is no consensus about
the management of asymptomatic gallstones in children. In a prospective
study of children with non-pigmented gallstones, Bruch SW, et al.
followed 41 children with non-specific or no symptoms for 21 months(51).
Of these, 50% remained or became asymptomatic, 32% experienced definite
improvement in symptoms, 18% had continued symptoms but none had any
biliary complications. Wesdorp, et al.(11) have substantiated this
observation in their study of 82 children with cholelithiasis who were
followed up for a mean period of 4.6 years. The suggested treatment
algorithm for gallstones in children is given in Fig.1.
Children with gallstones should be divided into two groups. Those with
typical symptoms (right upper quadrant or epigastric pain, nausea,
vomiting and fatty food intolerance) should have their gallbladders
removed. Asymptomatic children or children with nonspecific symptoms can
undergo safe follow up. These children will require observation into
adulthood to determine their lifetime risk of developing symptoms.
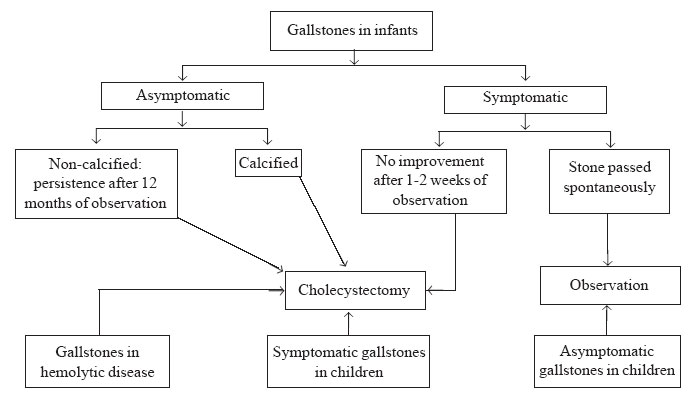 |
|
Fig. 1. Management algorithm for gallstones
in children. |
In recent years, laparoscopic cholecystectomy has
become the treatment of choice in the surgical management of children with
cholelithiasis. It has the advantage of being less invasive with lower
morbidity and mortality and shorter hospital stay over conventional open
cholecystectomy(52).
The role of dissolution therapy in the management of
gallstones in children remains to be defined. The only study of UDCA
therapy for gallstones in children by Gamba, et al.
have shown disappointing results(53). Of 15 children with radiolucent
stones (<10 mm) and a functional gallbladder treated for one year, stones
disappeared completely in only two cases but returned later in both.
Extracorporeal shock-wave lithotripsy for gallstones in
children has been successful in a single case report(54).
The approach to cholelithiasis in infancy is different as spontaneous
resolution has been reported in a significant proportion of cases (cholelithiasis
almost 50% and choledocholithiasis 30%)(44,45,55). Spontaneous resolution
within 6 months is more common with idiopathic gallstones than in patients
with known predisposing factors. Asymptomatic infants with idiopathic
cholelithiasis be observed for spontaneous resolution. Even for
choledocholithiasis, an observation period of 1-2 weeks is recommended
before active therapy as there is a chance of spontaneous resolution.
Cholecystectomy is indicated for symptomatic cholelithiasis, asymptomatic
choleli-thiasis persisting beyond 12 months and radiopaque calculi(44,45).
Management of cholelithiasis in hemolytic disease
In this group of children, screening with US is
recommended at around 5 years of age. Screening is also recommended before
splenectomy as both splenectomy and cholecystectomy can be combined in
presence of gallstones and it confers a survival benefit over splenectomy
alone in hereditary spherocytosis(56). However, there is no advantage of
doing cholecystectomy with splenectomy if there are no gallstones as these
patients are not at an increased risk of cholelithiasis after
splenec-tomy(57). In sickle-cell disease, prophylactic cholecystectomy is
recommended even for asympto-matic gallstones as it is difficult to
differentiate an acute abdominal crisis from acute cholecystitis, and the
morbidity and mortality of emergency cholecystectomy in this setting is
much higher than in elective cholecystectomy(58). To avoid sickling during
the perioperative period it is recommended that hemoglobin S be decreased
to at least 30% and total hemoglobin be increased to at least 11 g/dL.
During the surgery and recovery period, hypotension, dehydration, hypoxia,
hypothermia and acidosis should be prevented(47).
Choledocholithiasis
Most often common bile duct (CBD) stones are associated
with gallstones except in hemolytic conditions where these may be the
primary stones. Overall CBD stones are uncommon in children (only 10% of
all gallstones)(16,59), but infants have a higher incidence(45).
Clinical presentation of CBD stones comprises jaundice, cholangitis, and
gallstone pancreatitis. A CBD stone should also be suspected in a patient
with gallstones with hyperbilirubinemia (total bilirubin >1.3 mg/dL)
and/or a dilated CBD on US (>6 mm). The most appropriate method of
investigation and management of CBD stones seems to be laparoscopic
cholecystectomy (LC) with intraoperative cholangiogram (IOC) followed by
ERCP(60). In a study of 48 cases of suspected CBD stones, Mah, et al.
have compared preoperative ERCP followed by LC with LC+IOC followed by
ERCP(61). The diagnostic yield of ERCP in detecting CBD stone was just 23%
with the former approach compared with 100% with the latter approach.
|
Key Messages
• Gallstone disease is uncommon in children but
more cases are being diagnosed due to increasing use of
ultrasonography.
• Gallstones in infants most often resolve
spontaneously.
• Gallstones in a setting of hemolytic disease
develop after 5 years of age and require prophylactic
cholecystectomy.
• Asymptomatic gallstones or gallstones with
atypical symptoms may be observed as the natural history is benign. |
References
1. Rome Group for the Epidemiology and Prevention of
Cholelithiasis (GREPCO). The Epidemiology of gallstone disease in Rome,
Italy. Part I. Prevalence data in men. Hepatology 1988; 8: 904-906.
2. Bainton D, Davies GT, Evans KT, Gravelle IH.
Gallbladder disease. Prevalence in a South Wales Industrial Town. N Engl J
Med 1976; 294: 1147-1149.
3. Khuroo MS, Mahajan R, Zargar SA, Javid G, Sapru S.
Prevalence of biliary tract disease in India: a sonographic study in adult
population in Kashmir. Gut 1989; 30: 2001-2005.
4. Singh V, Trikha B, Nain CK, Singh K, Bose SM.
Epidemiology of gallstone disease in Chandigarh: A community-based study.
J Gastroenterol Hepatol 2001; 16: 560-563.
5. Malhotra SL. Epidemiological study of cholelithiasis
among railroad workers in India with special reference to causation. Gut
1968; 9: 290-295.
6. Kotwal MR, Rinchen CZ. Gallstone disease in the
Himalayas (Sikkim and North Bengal): causation and stone analysis. Indian
J Gastroenterol 1998; 17: 87-89.
7. Jayanthi V. Pattern of gallstone disease in Madras
city, south India-a hospital based survey. J Assoc Physicians India 1996;
44: 461-464.
8. Khan HN. Asymptomatic gallstones in the
laparo-scopic era. J R Coll Surg Edin Irel 2004; 2: 115.
9. Gracie WA, Ransohoff DF. The natural history of
silent gallstones: the innocent gallstone is not a myth. N Engl J Med
1982; 307: 798-800.
10. Schirmer WJ, Grisoni Er, Gauderer MWL. The spectrum
of cholelithiasis in the first year of life. J Pediatr Surg 1989; 24:
1064-1067.
11. Wesdorp I, Bosman D, de Graaff A, Aronson D, van
der Blij F, Taminiau J. Clinical presentations and predisposing factors of
cholelithiasis and sludge in children. J Pediatr Gastroenterol Nutr 2000;
31: 411-417.
12. Palasciano G, Portincasa P, Vinciguerra V, Velardi
A, Tardi S, Baldassarre G, et al. Gallstone prevalence and
gallbladder volume in children and adolescents: an epidemiological
ultrasonographic survey and relationship to body mass index. Am J
Gastroenterol 1989; 84: 1378-1382.
13. Nomura H, Kashiwagi S, Hayashi J, Kajiyama W,
Ikematsu H, Noguchi A, et al. Prevalence of gallstone disease in a
general population of Okinawa, Japan. Am J Epidemiol 1988; 128: 598-605.
14. Ganesh R, Muralinath S, Sankarnarayanan VS,
Sathiyasekaran M. Prevalence of cholelithiasis in children – a
hospital-based observation. Indian J Gastroenterol 2005; 24: 85.
15. Kaechele V, Wabitsch M, Thiere D, Kessler AL,
Haenle MM, Mayer H, et al. Prevalence of gallbladder stone disease
in obese children and adolescents: influence of the degree of obesity, sex
and pubertal development. J Pediatr Gastroenterol Nutr 2008;
42: 66-70.
16. Schweizer P, Lenz MP, Kirschner HJ. Pathogenesis
and symptomatology of cholelithiasis in childhood. Dig Surg 2000; 17:
459-467.
17. Webb DK, Darby JS, Dunn DT, Terry SI, Serjeant GR.
Gallstones in Jamaican children with homozygous sickle-cell disease. Arch
Dis Child 1989; 64: 693-696.
18. Tripathy D, Dash BP, Mohapatra BN, Kar BC.
Cholelithiasis in sickle cell disease in India. J Assoc Physicians India
1997; 45: 287-289.
19. do Santos Gumiero AP, Bellomo-Brandao MA,
Costa-Pinto EAL. Gallstones in children with sickle cell disease followed
up at a Brazilian hematology center. Arq Gastroenterol 2008; 45:
313-318.
20. Croom RD 3rd, McMillan CW, Orringer EP, Sheldon GF.
Hereditary spherocytosis. Recent experience and current concept of patho-physiology.
Ann Surg 1986; 203: 34-39.
21. Kar R, Rao S, Srinivas UM, Mishra P, Pati HP.
Clinico-hematological profile of hereditary spherocytosis: experience from
a tertiary care center in North India. Hematology 2009; 14: 164-167.
22. Kalayci AG, Albayrak D, Gunes M, Incesu L, Agac R.
The incidence of gallbladder stones and gallbladder function in beta-thalassemic
children. Acta Radiol 1999; 40: 440-443.
23. Lotfi M, Keramati P, Assadsangabi R, Nabavizadeh
SA, Karimi M. Ultrasonographic assessment of the prevalence of
cholelithiasis and biliary sludge in beta-thalassemia patients in Iran.
Med Sci Monit 2009; 15; CR398-402.
24. Origa R, Galanello R, Perseu L, Tavazzi D,
Cappellini MD, Terenzani L, et al. Cholelithiasis in thalassemia
major. Eur J Hematol 2008; 82: 22-25.
25. Galanello R, Piras S, Barella S, Leoni GB,
Cipollina MD, Perseu L, et al. Cholelithiasis and Gilbert’s
syndrome in homozygour beta-thalassemia. Br J Haematol 2001; 115: 926-928.
26. Chawla Y, Sarkar B, Marwaha RK, Dilawari JB.
Multitransfused children with thalassemia major do not have gallstones.
Trop Gastroenterol 1997; 18: 107-108.
27. Hussaini SH, Pereira SP, Veysey MJ, Kennedy C,
Jenkins P, Murphy GM, et al. Roles of gall bladder emptying and
intestinal transit in the pathogenesis of octreotide induced gall bladder
stones. Gut 1996; 38: 775-783.
28. Jawaheer G, Pierro A, Lloyd DA, Shaw NJ.
Gallbladder contractility in neonates: effects of parenteral and enteral
feeding. Arch Dis Child Fetal Neonatal Ed 1995; 72: F200-202.
29. Roslyn JJ, Berquist WE, Pitt HA, Mann LL, Kangarloo
H, DenBesten L, Ament ME. Increased risk of gallstones in children
receiving total parenteral nutrition. Pediatrics 1983; 71: 784-789.
30. Matos C, Avni EF, Van Gansbeke D, Pardou A,
Struyven J. Total parenteral nutrition (TPN) and gallbladder diseases in
neonates - sonographic assessment. J Ultrasound Med 1987; 6:
243-248.
31. Schaad UB, Tschappeler H, Lentze MJ. Transient
formation of precipitations in the gallbladder associated with ceftriaxone
therapy. Pediatr Infect Dis 1986; 5: 708-710.
32. Richards DM, Heel RC, Brogden RN, Speight TM, Avery
GS. Ceftriaxone. A review of its antibacterial activity, pharmacological
properties and therapeutic use. Drugs 1984; 27: 469-527.
33. Shiffman ML, Keith FB, Moore EW. Pathogenesis of
ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone
binding and solubility. Gastroenterology 1990; 99: 1772-1778.
34. Lee SP, Lipsky BA, Teefey SA. Gallbladder sludge
and antibiotics. Pediatr Infect Dis J 1990; 9: 422-423.
35. Schaad UB, Wedgwood-Krucko J, Tschaeppeler H.
Reversible ceftriaxone-associated biliary pseudolithiasis in children.
Lancet 1988; 2: 1411-1413.
36. Schaad UB, Suter S, Gianella-Borradori A,
Pfenninger J, Auckenthaler R, Bernath O, et al. A comparison of
ceftriaxone and cefuroxome for the treatment of bacterial meningitis in
children. N Engl J Med 1990; 322: 141-147.
37. Rosmorduc O, Poupon R. Low phospholipid associated
cholelithiasis: association with mutation in the MDR3/ABCB4 gene. Orphanet
J Rare Dis 2007; 2: 29.
38. Rosmorduc O, Hermelin B, Poupon R. MDR3 gene defect
in adults with symptomatic intrahepatic and gallbladder cholesterol
cholelithiasis. Gastro-enterology 2001; 120: 1449-1467.
39. Beretsky I, Lankin DH. Diagnosis of fetal
cholelithiasis using real-time high resolution imaging employing digital
detection. J Ultrasound Med 1983; 2: 381-383.
40. Suma V, Marini A, Bucci N, Toffolutti T, Talenti E.
Fetal gallstones: sonographic and clinical observations. Ultrasound Obstet
Gynecol 1998; 12: 439-441.
41. Brown LD, Teele LR, Doubilet MP. Echogenic material
in the fetal gallbladder: sonographic and clinical observations. Radiology
1992; 182: 73-76.
42. Abbitt LP, Mc Ilhenuy J. Prenatal detection of
gallstones. J Clin Ultrasound 1990; 18: 202-204.
43. Stringer MD, Lim P, Cave M, Martinez D, Lilford RJ.
Fetal gallstones. J Pediatr Surg 1996; 31: 1589-1591.
44. St-Vil D, Yazbeck S, Luks FI, Hancock BJ,
Filiatrault D, Youssef S. Cholelithiasis in newborns and infants. J
Pediatr Surg 1992; 27: 1305-1307.
45. Debray D, Pariente D, Gauthier F, Myara A, Bernard
O. Cholelithiasis in infancy: A study of 40 cases. J Pediatr 1993; 122:
385-391.
46. Rief S, Sloven DG, Lebenthal E. Gallstones in
children. Am J Dis Child 1991; 145: 105-108.
47. Holcomb GW Jr, Holcomb GW III. Cholelithiasis in
infants, children and adolescents. Pediatric Rev 1990; 11: 268-274.
48. Millar AJW. Surgical disorders of the liver and
bile ducts and portal hypertension. In: Kelly DA editors, Disease
of the liver and biliary system in children, 3 rd
Edition, Wiley-Blackwell publication UK, 2008, pp 433-474.
49. Poddar U, Thapa BR, Bhasin DK, Prasad A, Nagi B,
Singh K. Endoscopic retrograde cholangio-pancreatography in the management
of pancreatobiliary disorders in children. J Gastroenterol Hepatol 2001;
16: 927-931.
50. Prasad H, Poddar U, Thapa BR, Bhasin DK, Singh K.
Endoscopic management of post laparoscopic cholecystectomy bile leak in a
child. Gastrointest Endosc 2000; 51: 506-507.
51. Bruch SW, Ein SH, Rocchi C, Kim PCW. The management
of nonpigmented gallstones in children. J Pediatr Surg 2000; 35: 729-732.
52. Chan S, Currie J, Malik AI, Mahomed AA. Pediatric
cholecystectomy: shifting goalposts in the laparoscopic era. Surg Endosc
2008; 22: 1392-1395.
53. Gamba PG, Zancan L, Muraca M, Vilei MT, Talenti E,
Guglielmi M. Is there a place of medical treatment in children with
gallstones? J Pediatr Surg 1997; 32: 476-478.
54. Sokal EM, De Bilderling G, Clapuyt P, Opsomer RJ,
Buts JP. Extracorporeal shock-wave lithotripsy for calcified lower
choledocholithiasis in an 18-month-old boy. J Pediatr Gastroenterol Nutr
1994; 18: 391-394.
55. Miltenburg DM, Schaffer R, Breslin T, Brandt ML.
Changing indications for pediatric cholecystec-tomy. Pediatrics 2000; 105:
1250-1253.
56. Marchetti M, Quaglini S, Barosi G. Prophylactic
splenectomy and cholecystectomy in mild here-ditary spherocytosis:
analyzing the decision in different clinical scenarios. J Intern Med 1998;
244: 217-226.
57. Sandler A, Winkel G, Kimura K, Soper R. The role of
prophylactic cholecystectomy during splenec-tomy in children with
hereditary spherocytosis. J Pediatr Surg 1999; 34: 1077-1078.
58. Al-Salem AH. Should cholecystectomy be performed
concomitantly with splenectomy in children with sickle-cell disease?
Pediatr Surg Int 2003; 19: 71-74.
59. Newman KD, Powell DM, Holcomb GW III. The
management of choledocholithiasis in children in the era of laparoscopic
cholecystectomy. J Pediatr Surg 1997; 32: 1116-1119.
60. Vrochides DV, Sorrells DL, Kurkchubasche AG,
Wesselhoeft CW Jr, Tracy TF Jr, Luks FI. Is there a role for routine
preoperative endoscopic retrograde cholangiopancreatography for suspected
choledo-cholithiasis in children? Arch Surg 2005; 140: 359-361.
61. Mah D, Wales P, Njere I, Kortan P, Masiakos P, Kim CW. Management
of suspected common bile duct stones in children: role of selective
intraoperative cholangiogram and endoscopic retrograde
cholangiopancreatography. J Pediatr Surg 2004; 39: 808-812.
|
|
|
 |
|

