|
|
|
Indian Pediatr 2010;47: 937-943 |
 |
Reactive Thrombocytosis in Febrile Young
Infants with Serious Bacterial Infection |
|
S Fouzas, L Mantagou, E Skylogianni and A Varvarigou
From the Department of Pediatrics, University Hospital of
Patras, Patras, Greece.
Correspondence to: Fouzas Sotirios, Department of
Pediatrics, University Hospital of Patras, Rio,
Patras, 265 04, Greece.
Email: [email protected]
Published online 2010 March 15.
PII:
S097475590900522-1
|
|
Abstract
Objective: To estimate the incidence of reactive
thrombocytosis among febrile young infants and to asses the utility of
platelet count as a potential predictor of serious bacterial infection (SBI).
Design: Retrospective study between January 2005
and December 2008.
Setting: Tertiary care pediatric unit.
Participants: All infants 29 to 89 days of age,
admitted with rectal temperature >38oC without a focus of infection.
Main Outcome Measures: The results of the sepsis
evaluation on admission were recorded. SBI included all cases of occult
bacteremia, urinary tract infection, bacterial meningitis, pneumonia,
bacterial gastroenteritis and infections of the soft tissues and bones.
Results: Of the 408 infants studied, 103 (25.2%)
had SBI. Platelet count was significantly higher in infants with SBI
compared to those without (median 513000 /mm3 [interquartile
range 455,000–598,000/mm3] vs median 398000/mm3;
[interquartile range 313,000–463,000/mm3]; P<0.001).
Thrombocytosis had only moderate ability in predicting SBI (area under
the curve: 0.74, 95%CI 0.70-0.79). The combination of platelet count
³450,000/mm3,
WBC ³15,000/mm3,
C-reactive protein ³2
mg/dL, and pyuria ³10
WBC/hpf would lead to misclassification of 4 infants with SBI (3.9% of
SBIs; negative likelihood ratio 0.08).
Conclusions: Reactive thrombocytosis was a
frequent finding in young infants with SBI. Thrombocytosis
³450,000 cells/mm3, in combination
with leucocytosis, elevated CRP and pyuria, may help in early
recognition of febrile young infants at risk for SBI.
Key words: Diagnosis, Fever, Infants, Serious bacterial
infection, Thrombocytosis.
|
|
F
ebrile infants less than 3 months
of age present a management challenge, as many of these have no
identifiable source of fever, and the prevalence of serious bacterial
infection (SBI) in this age group is high(1-7). The most commonly
suggested strategy is for the febrile neonates to be admitted to a
hospital and undergo full sepsis workup(5-7). In the past decade, several
management strategies based on the combination of physical and laboratory
findings have been proposed, but no protocol has been universally
adopted(8-11). Furthermore, a series of laboratory parameters such as
white blood cell (WBC) count, absolute neutrophil count, pyuria,
C-reactive protein (CRP), and more recently, interleukin-6 and
procalcitonin, have been extensively evaluated and compared as potential
predictors of SBI(8-16). These laboratory tests lack adequate predictive
ability and the idea of a simple, rapid and inexpensive diagnostic test
that could accurately identify bacterial infections among febrile infants,
remains unattainable(3,4,6, 13,15,17).
Reactive thrombocytosis is a common finding in infants
that occurs in the preponderance of cases secondary to an
infection(18-25). To our knowledge, no study has previously focused on the
incidence and characteristics of reactive thrombocytosis in young infants
with SBI. Moreover, the platelet count has neither been considered nor
evaluated as a potential predictor of SBI among young febrile infants.
The objective of our study was to estimate the
incidence of reactive thrombocytosis in febrile young infants, especially
in those with bacterial infections, and assess the value of platelet count
as a potential predictor of SBI.
Methods
We retrospectively reviewed the case-records of infants
aged 29 to 89 days, admitted to our tertiary care pediatric unit between 1
January 2005 and 31 December 2008 for investigation of fever (defined as
rectal temperature >38 oC) without a
focus of infection. Infants who had fever for more than 72 hours, and had
received antibiotics or vaccination within 48 hours of presentation, were
excluded.
All patients had sepsis evaluation including WBC count,
platelet count, blood culture, urine microscopy and culture and CRP.
Lumbar puncture for cerebrospinal fluid (CSF) analysis and culture, as
well as stool culture and chest radiographs, were obtained at the
discretion of the attending pediatrician.
The WBC count with differential and the platelet count
were quantified using automated laboratory equipment (Sysmex SE 9500, GMI,
Inc). Blood cultures were monitored by an automated system (BacT/ALERT 3D,
bioMérieux, Inc). Urine was obtained by suprapubic needle aspiration or by
urethral catheterization using a sterile technique. The WBC in the urine
were quantified by standard microscopic examination
and expressed as WBC per high power field (hpf)(28). The urine, CSF and
stool cultures were monitored using standard laboratory techniques.
Serious bacterial infection (discharge diagnosis) was
defined as occult bacteremia, urinary tract infection (UTI), bacterial
meningitis, pneumonia, bacterial enteritis and infection of soft tissue or
bones. Isolates such as Staphylococcus epidermidis or
Streptococcus viridans in the blood culture were considered
contaminants unless they were isolated from more than two consecutive
cultures. Urinary tract infection was defined as a single known pathogen
growth ³1000
colony-forming units (cfu) /mL of urine obtained by suprapubic needle
aspiration or ³100,000
cfu /mL of urine obtained by urethral catheterization. Pneumonia was
defined as the presence of a focal infiltrate on chest radiograph as
interpreted by the attending radiologist(29).
The data were analyzed using the SPSS 15.0 for Windows
(SPSS, Inc). Non parametric data are presented as medians with
interquartile ranges (IQR). Differences between the groups were assessed
for statistical significance using either the Mann Whitney U or
chi-squared test, as appropriate. Individual differences between
nonparametric variables were evaluated by the Kruskal-Wallis
multiple-comparison z-value test with Bonferroni correction (alpha=0.05;
medians significantly different if z-value >2.93), using the statistical
package NCSS 2004 (Number Cruncher Statistical Systems, Kaysville, UT,
USA). The overall performance of individual parameters in predicting SBI
was assessed by receiver operating characteristic (ROC) curve analyses and
area under the curve (AUC) comparisons, using the statistical software
MedCalc 8.1 (MedCalc, Mariakerke, Belgium). The study was approved by the
ethics committee of the University Hospital of Patras, Greece.
Results
During the study period, 464 infants 29 to 89 days of
age, were admitted for investigation of fever >38 oC
without a source. Of these, 12 had fever for more than 72 hours, 9 had
received vaccination, 23 were treated with antibiotics within 48 hours of
presentation, and 12 had incomplete medical records.
Of the remaining 408 infants, SBI was documented in 103
(25.2%). Of these, 88 (85.4%) had UTI (74 with Esherichia coli), 9
occult bacteremia (2 with Streptococcus pneumoniae, 3 with Group
B Streptococcus, 2 with Staphylococcus aureus and 2 with
Esherichia coli), 6 infants had pneumonia, and 2 were diagnosed with
bacterial meningitis (1 with Neisseria meningitidis and 1 with
Group B Streptococcus). Two infants had concurrent positive blood and
urine cultures for Esherichia coli. None of the infants with
pneumonia had documented bacteremia. The remaining 305 infants (74.8%)
with negative sepsis evaluation were categorized in the non–SBI group.
Clinical and laboratory characteristics of the non–SBI
and SBI groups are presented in Table I. A comparison of
platelet counts between the non–SBI and SBI groups is shown in Table
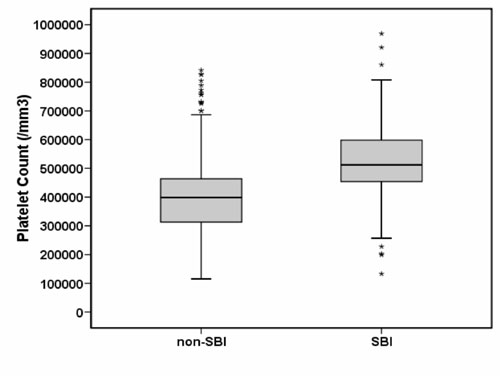
II. We also noted a substantial overlap between the two groups (Fig.
1).
TABLE I
Clinical and Laboratory Characteristics of the Non-SBI and SBI Groups
| |
Non-SBI (n=305) |
SBI (n=103) |
P Value |
|
Age (d) |
57 (42-72) |
60 (44–75) |
0.10 |
|
Sex (male/female) |
162/143 |
57/46 |
0.78 |
|
Duration of fever (h) |
14 (6–27) |
14 (6–29) |
0.49 |
|
Fever on admission (oC) |
38.5 (38.1–38.8) |
38.5 (38.1-39.0) |
0.22 |
|
Hemoglobin (g/dL) |
10.5 (9.7–11.0) |
10.4 (9.6–11.0) |
0.30 |
|
WBC (103/mm3) |
9.65 (7.15–14.20) |
16.0 (11.1–20.2) |
<0.0001 |
|
PLT (103/mm3) |
398 (313–463) |
513 (455–598) |
<0.0001 |
|
CRP (mg/dL) |
0.2 (0.0–1.2) |
1.6 (0.1-–4.2) |
<0.0001 |
|
Pyuria (WBC/hpf) |
1 (1–3) |
10 (3–45) |
<0.0001 |
|
Data are expressed as median (interquartile range); All
comparisons by Mann-Whitney U test except sex difference by
x2 test; SBI: serious bacterial infection; WBC: white blood count;
PLT: platelet count; CRP: C-reactive protein; hpf: high power field. |
 |
|
Fig. 1 Box plots presenting the
distribution of platelet counts in the non–SBI and SBI group. The
central box represents the values from the lower to upper quartile
(25 th to 75th
percentile). The middle line represents the median. A line extends
from the minimum to the maximum value, excluding "outside" values or
"outliers" which are displayed as separate points. |
TABLE II
Platelet Counts in the Non–SBI Group and SBI Subgroups
|
Group* |
N |
Platelet count (103/mm3) |
Significant to: ‡ |
|
|
|
Median |
IQR |
(z-value) |
|
|
|
|
|
UTI (7.85) |
|
non–SBI |
305 |
398 |
313–463 |
OB (3.95) |
|
|
|
|
|
PN (3.23) |
|
UTI |
88† |
513 |
453–597 |
non–SBI (7.85) |
|
OB |
9† |
523 |
500–611 |
non–SBI (3.95) |
|
PN |
6 |
490 |
477–541 |
non-–SBI (3.23) |
|
IQR, interquartile range; SBI: serious bacterial infection; UTI:
urinary tract infection; OB: occult bacteremia; PN: pneumonia* Infants
with Bacterial meningitis (n=2) were not included in comparison; †
four UTIs with concomitant bacteremia considered as OB; ‡ Kruskal-Wallis
multiple-comparison z-value test with Bonferroni correction (for
alpha=0.05 medians are considered significantly different if z-value
>2.93). |
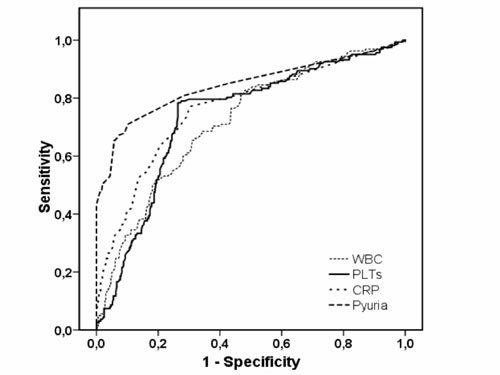
The ROC curve depicting the ability of platelet count
in identifying infants with SBI was also compared with WBC, CRP and pyuria
(Fig. 2).
 |
|
Fig. 2 Receiver operating characteristics
curve for PLT, WBC, CRP and pyuria predicting serious bacterial
infection in febrile young infants. Area under the curve (AUC) for
PLT 0.74 (95%CI: 0.70–0.79); for WBC 0.72 (95%CI: 0.67–0.76); for
CRP 0.75 (95%CI: 0.71–0.80); and for pyuria 0.82 (95%CI: 0.78–0.86).
The AUC for WBC was significantly lower compared to the AUC for
pyuria (P=0.02). No statistically significant differences were found
between the AUCs of the other parameters. WBC white blood
count; PLT platelets count; CRP C-reactive protein. |
To explore further the utility of platelet count in
identifying SBI, test characteristics were calculated for different
decision thresholds (Table III). A platelet count of
³450,000/mm3
had the highest accuracy for identifying high-risk infants. At this
decision threshold, 18 infants with SBI (17.4% of SBIs) were falsely
classified as low-risk and 90 infants without SBI (22.0% of the study
population) were falsely classified as high risk (negative LR 0.25;
positive LR 2.8).
TABLE III
Test Characteristics for Different Platelet Count Thresholds
|
Platelet threshold |
n |
SBI |
Sensitivity |
Specificity |
PPV* |
NPV* |
LR + |
LR – |
|
(103/mm3) |
|
(n) |
(%) |
(%) |
(%) |
(%) |
|
|
|
≥400 |
253 |
88 |
85.4 |
45.9 |
34.8 |
90.3 |
1.6 |
0.32 |
|
≥450 |
175 |
85 |
82.5 |
70.5 |
48.6 |
92.3 |
2.8 |
0.25 |
|
≥500 |
122 |
54 |
52.4 |
77.7 |
44.3 |
82.9 |
2.4 |
0.61 |
|
≥600 |
53 |
23 |
22.3 |
90.2 |
43.4 |
77.5 |
2.3 |
0.86 |
|
* The prevalence of SBI was 25.2% (103/408
infants); SBI: serious bacterial infection; PPV: positive predictive
value; NPV: negative predictive value; LR + likelihood ratio for
positive test; LR – likelihood ratio for negative test. |
A combined high-risk criterion of
³15,000
/mm3 for WBC and
³10
WBC/hpf for pyuria, led to the misclassification of 17.5% of the SBIs (18
infants; negative LR 0.24), while 20.8% were falsely classified as
high-risk (85 infants; positive LR 3.0). Further combination of WBC
³15000
/mm3, pyuria
³10
WBC /hpf, and CRP ³2
mg/dl, led to the misclassification of 9 infants with SBI (8.7% of SBIs;
negative LR 0.16), whereas 135 infants without bacterial infection (33.1%
of the population) were falsely classified as high-risk (positive LR 2.1).
This 12.3% increase in the percentage of the falsely classified high-risk
infants was significant (P<0.001). The addition to the above
criteria of a platelet count of
³450,000/mm3,
resulted in a decrease of the percentage of the misclassified SBIs to 3.9%
(4 infants; negative LR 0.08), and an insignificant increase (3.6%; P=0.31)
of the infants falsely classified as high-risk to approximately 36.7% (150
infants; positive LR 2.0) (Table IV).
TABLE IV
Test Characteristics for Different Decision Thresholds
|
Decision threshold |
Sensitivity (%) |
Specificity (%) |
PPV* (%) |
NPV* (%) |
LR+ |
LR– |
|
WBC >15×103/mm3 |
52.4 |
78.7 |
45.4 |
83.0 |
2.5 |
0.6 |
|
Pyuria ≥10 WBC/hpf |
65.0 |
94.1 |
78.8 |
88.9 |
11.0 |
0.37 |
|
PLT ≥450×103/mm3 |
82.5 |
70.5 |
48.6 |
92.3 |
2.8 |
0.25 |
|
CRP ≥2 mg/dL |
51.5 |
86.6 |
56.4 |
84.1 |
3.8 |
0.56 |
|
WBC + pyuria |
82.5 |
72.1 |
50.0 |
92.4 |
3.0 |
0.24 |
|
WBC + pyuria + CRP |
91.3 |
55.7 |
41.0 |
95.0 |
2.1 |
0.16 |
|
WBC + pyuria + CRP + PLT |
96.1 |
50.8 |
39.8 |
97.5 |
2.0 |
0.08 |
|
* The prevalence of SBI was 25.2% (103/408
infants) PPV: positive predictive value; NPV: negative predictive
value; LR+: likelihood ratio for positive test; LR–: likelihood
ratio for negative test; SBI serious bacterial infection; WBC white
blood count; PLT platelets count; CRP C-reactive protein; hpf high
power field. |
Discussion
In this study, platelet count was significantly higher
in febrile infants with documented bacterial infection, particularly in
those with UTI, occult bacteremia and pneumonia. However, due to a
substantial overlap, it was difficult to identify a threshold value that
could clearly differentiate infants with SBI from other febrile infants.
Platelet counts of ³450,000/mm3
had the highest accuracy in differentiating infants with SBI, with less
false negative and false positive results. The overall ability of platelet
count to identify infants with SBI was moderate (AUC 0.74), but comparable
to the other parameters.
The prevalence of SBI in our population (25.2%) was
quite high. This study was conducted in a tertiary care pediatric unit
that represents the referral center for south-western Greece. Thus, only
infants who were more ill appearing or presumably more likely to have SBI
may have been referred to our unit. In fact, an appreciable percentage of
well-appearing febrile infants are evaluated in primary and secondary
pediatric care facilities of our region. A larger, prospective and
multicenter study would yield an unbiased prevalence of SBI among young
febrile infants without a source of infection and would allow for a more
reliable evaluation of the predictive ability of reactive thrombocytosis.
The fact that platelets can behave like an acute phase
reactant is well recognized(18-25). Stimulation of platelet production is
triggered by interleukin-6 which enhances megakaryopoiesis directly and
indirectly by stimulating hepatic thrombopoietin production(18,23). Yet,
the role of reactive thrombocytosis, especially in the sphere of the
immature immune system of young infants, needs to be further elucidated.
In addition, thrombocytosis secondary to anemia is a matter of concern in
this age group(18-21). In this study, platelet count was significantly
higher in infants with SBI compared to those without, and this was
independent to the incidence of anemia in the two groups. Reactive
thrombocytosis in combination with WBC, CRP and pyuria seems to be a
useful tool that could help clinician to target further investigation and
follow-up strategy.
Contributors: SF developed the concept,
helped in data collection, performed data analysis, and prepared the
manuscript. LM and ES performed data collection and helped in the
preparation and correction of the manuscript. AV developed the concept,
interpreted the results and revised the manuscript for important
intellectual content. She will act as guarantor of the study. The final
manuscript was approved by all authors.
Funding: None.
Competing interest: None stated.
|
What is Already Known?
•
Reactive thrombocytosis is common in infants with bacterial
infections.
What this Study Adds?
•
Thrombocytosis ≥450,000 cells/mm3 may help in assessing
the risk for serious bacterial infection in febrile young infants. |
References
1. Slater M, Krug SE. Evaluation of the infant with
fever without source: an evidence based approach. Emerg Med Clin North Am
1999; 17: 97-126.
2. Baraff LJ, Oslund SA, Schriger DL, Stephen ML.
Probability of bacterial infections in febrile infants less than three
months of age: a metaanalysis. Pediatr Infect Dis J 1992; 11: 257-264
3. Isaacman DJ, Shults J, Gross TK, Davis PH, Harper M.
Predictors of bacteremia in febrile children 3 to 36 months of age.
Pediatrics 2000; 106: 977-982.
4. Hsiao AL, Chen L, Baker MD. Incidence and predictors
of serious bacterial infection among 57- to 180-day-old infants.
Pediatrics 2006; 117: 1695-1701.
5. Baker MD, Avner JR, Bell LM. Failure of infant
observation scales in detecting serious illness in febrile, 4- to 8-
week-old infants. Pediatrics 1990; 85: 1040-1043.
6. Steere M, Sharieff GQ, Stenklyft PH. Fever in
children less than 36 months of age – Questions and strategies for
management in the emergency department. J Emerg Med 2003; 25: 149-157.
7. Ishimine P. Fever without source in Children 0 to 36
months of age. Pediatr Clin North Am 2006; 53: 167-194.
8. Baraff LJ, Bass JW, Fleisher GR, Klein JO, McCracken
GH Jr, Powell KR, et al. Practice guideline for the management of
infants and children 0 to 36 months of age with fever without a source.
Pediatrics 1993; 92: 1-12.
9. Baskin MN, O’Rourke EJ, Fleisher GR. Outpatient
treatment of febrile infants 28 to 89 days of age with intramuscular
administration of ceftriaxone. J Pediatr 1992; 120: 22-27.
10. Baker MD, Bell LM, Avner JR. Outpatient management
without antibiotics of fever in selected infants. N Engl J Med 1993; 329:
1437-1441.
11. Jaskiewicz JA, McCarthy CA, Richardson AC, White
KC, Fisher DJ, Dagan R, et al. Febrile Infant Collaborative Study
Group. Febrile infants at low risk for serious bacterial infection – an
appraisal of the Rochester criteria and implications for management.
Pediatrics 1994; 94: 390-396.
12. Bachur RG, Harper MB. Predictive model for serious
bacterial infections among infants younger than 3 months of age.
Pediatrics 2001; 108: 311-316.
13. Pulliam PN, Attia MW, Cronan KM. C-reactive protein
in febrile children 1 to 36 months of age with clinically undetectable
serious bacterial infection. Pediatrics 2001; 108; 1275-1279.
14. Galetto-Lacour A, Zamora SA, Gervaix A. Bedside
procalcitonin and C-reactive protein tests in children with fever without
localizing signs of infection seen in a referral center. Pediatrics 2003;
112; 1054-1060.
15. Mathew JL. Can CRP predict bacterial infection in
children with fever? Indian Pediatr 2008; 45:129-133.
16. Olaciregui I, Hernández U, Muñoz JA, Emparanza JI,
Landa JJ. Markers that predict serious bacterial infection in infants
under 3 months of age presenting with fever of unknown origin. Arch Dis
Child 2009; 94: 501-505.
17. Hsiao AL, Baker MD. Fever in the new millennium: a
review of recent studies of markers of serious bacterial infection in
febrile children. Curr Opin Pediatr 2005; 17: 56-61.
18. Mantadakis E, Tsalkidis A, Chatzimichael A.
Thrombocytosis in childhood. Indian Pediatr 2008; 45: 669-677.
19. Matsubara K, Fukaya T, Nigami H, Harigaya H, Hirata
T, Nozaki H, et al. Age-dependent changes in the incidence and
etiology of childhood thrombocytosis. Acta Haematol 2004; 111:132-137.
20. Dame C, Sutor AH. Primary and secondary
thrombocytosis in childhood. Br J Haematol 2005; 129: 165-177.
21. O’Shea J, Sherlock M, Philip R. Thrombocytosis in
childhood. Acta Haematol 2005; 113: 212.
22. Vlacha V, Feketea G. Thrombocytosis in pediatric
patients is associated with severe lower respiratory tract inflammation.
Arch Med Res 2006; 37: 755-759.
23. Dodig S, Raos M, Kovac K, Nogalo B, Benko B,
Glojnaric I, et al. Thrombopoietin and interleukin-6 in children
with pneumonia-associated thrombo-cytosis. Arch Med Res 2005; 36: 124-128.
24. Thomas GA, O’Brien RT. Thrombocytosis in children
with Hemophilus influenzae meningitis. Clin Pediatr (Phila) 1986;
25: 610-611.
25. Garoufi A, Voutsioti K, Tsapra H, Karpathios T,
Zeis PM. Reactive thrombocytosis in children with upper urinary tract
infections. Acta Paediatr 2001; 90: 448-449.
26. Dharnidharka VR, Kandoth PW. Prevalence of
bacteriuria in febrile infants. Indian Pediatr 1993; 30: 987-990.
27. Srivaths PR, Rath B, Prakash SK, Talukdar B.
Usefulness of screening febrile infants for urinary tract infection.
Indian Pediatr 1996; 33: 218-220.
28. Hoberman A, Wald ER. Urinary tract infections in
young febrile children. Pediatr Infect Dis J 1997; 16: 11-17.
29. Bachur R, Perry H, Harper M. Occult pneumonias:
empiric chest radiographs in febrile children with leukocytosis. Ann Emerg
Med 1999; 33: 166-173.
|
|
|
 |
|

