In 1993, WHO issued a standard
protocol to determine measles case fatality ratios
in a community(3). Community based studies provide
the best available data in the published literature
on measles CFR. Studies from Indian hospitals or
other health centers are likely biased, since
measles cases with complications are likely
oversampled(4). Passive surveillance (case report)
studies are also prone to under-reporting of measles
cases and deaths.
A recent measles CFR review of
community based studies was published by WHO in
2008; however, the authors did not perform an
in-depth analysis of CFRs for India(5). The most
recent review of measles CFR for India was published
in 1994(6). Since 1994, India has increased vaccine
coverage and routine vitamin A treatment was
introduced. Here we present our updated systematic
review of Indian CFR of measles from community based
studies published 1980-2008.
Methods
We systematically reviewed all
published literature from January 1, 1980 to
December 31, 2008 to identify Indian community based
measles studies with data on measles CFRs. PubMed,
Cochrane Libraries, and all World Health
Organization Regional Databases were searched in all
languages using combinations of the terms: India,
measles, case fatality, death,
and mortality. Prospective cohort and
cross-sectional studies were abstracted. Studies
were included if the study participants were from a
defined Indian population with data from 1975-2008.
Hospital or healthcare centre based studies and
passive surveillance were excluded since these
populations are likely not representative of the
general Indian population.
Measles disease and measles
attributed deaths were classified by the authors of
the included studies. Data abstracted from the
studies included: a location description
(State/Union territory and urban/rural study site),
year, type of study, if data were collected during
an outbreak, measles cases by age, and measles
deaths by age. We present CFR by the specified age
groups of <1 year, 1-4 years, 5-9 years and 10+
years in order to simplify study comparison.
However, if study data did not allow for these
groupings, we present the data as reported.
We first preformed a descriptive
analysis of the studies and investigated differences
by study location, outbreak setting, type of study,
and year. We report the median CFR by group and in
parenthesis report the 1st (Q1) and 3rd (Q3)
quartiles. In order to test differences between
groups, we utilized the Kruskal–Wallis test, a
non-parametric method for testing equality of
population medians among groups(7). Study year was
dichotomized by before and after 1994, as this was
the year the last Indian CFR review was published. A
P value <0.05 was considered statistically
significant for all analyses. Analyses were
conducted using STATA 10.0 Special Edition (STATACORP,
College Station, TX).
Results
We identified 25 Indian community
based measles CFR studies with data on 27 distinct
communities from 12 States/Union territories (8-32).
Two studies presented data for two distinct
populations and results were entered into the
database by population (26,28). Study descriptors
and results are presented for the 27 community
populations in Table I. Twenty of the
studies were cross-sectional (74.1%) and most were
conducted in rural areas (81.5%). In addition, most
of the studies were performed during measles
outbreaks (70.0%). A total of 8247 measles cases and
218 measles attributed deaths occurred in the
studies (pooled CFR=2.64%). The mean CFR was 4.27%
with a range of 0.00-31.25% and the median was 1.63
(Q1=0.00 and Q3=5.06).
Table I
Description and Results of Indian Community Based Studies Conducted From 1975 To 2008
Next, we analyzed the data for
factors associated with measles CFRs. The median CFR
for prospective studies was 1.91 (Q1=0.79 and Q3=
3.52), and 1.16 (Q1=0.00 and Q3=7.00) for
cross-sectional studies; the difference was not
significant (P=0.811). The CFRs for studies
conducted in rural communities (median=2.79, Q1=0.20
and Q3=7.00) were significantly higher in comparison
to urban studies (median=0.00, Q1=0.00 and Q3=0.00)
(P=0.015). The median CFR for studies
performed during measles outbreaks was 2.86 (Q1=0.00
and Q3=7.07), and 1.10 (Q1=0.00 and Q3=2.19) for
endemic settings; the difference was not significant
(P=0.183).
Only 6 studies with data on 7
populations separated measles CFR by age and as a
result we were unable to perform a statistical
analysis of trend by age; however, in Fig.1
we present a line graph of the data
(8,9,12,13,24,26). This graph suggests a decrease in
measles CFR with age, but whether CFRs for <1 yrs
and 1-4 yrs differ is not clear.
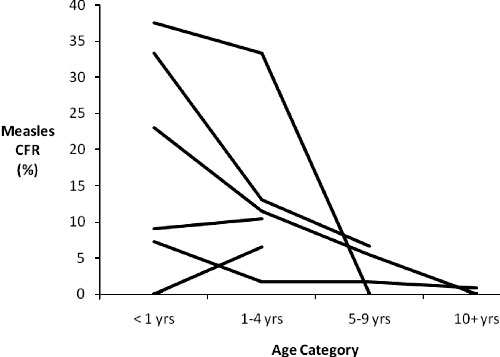 |
|
Fig.1 Measles case
fatality ratio by age category. |
We also assessed changes in
measles CFR over time. In Fig.1, we
present CFRs by midpoint study year, which suggests
a decline in CFR over time. We also determined that
CFR for studies occurring before 1994 (median=2.71,
Q1=0.20 and Q3=7.00) were significantly greater in
comparison to studies conducted after 1994
(median=0.00, Q1=0.00 and Q3=1.16) (P=0.031).
Discussion
Measles case fatality ratios are
known to significantly differ between countries and
vary within populations over time(5). We reviewed
Indian community based measles CFR studies to
investigate factors influencing CFR and changes in
mortality over time. CFR data are essential for
disease burden modeling and an updated review of CFR
was needed.
Measles CFR in India appears to
have decreased during 1975-2008. We hypothesize that
increased measles vaccination coverage in India is
the main factor contributing to this decline, in
addition to other factors including the introduction
of vitamin A in case management and increasing
vitamin A supplementation coverage. However, it may
not be appropriate to generalize from this review
that CFR has decreased for the entire population of
India, since published data are only available for
select communities in 12 Indian states or Union
territories. In addition, 38% of all districts in
India still had measles vaccine coverage less than
50% in 2005 and these districts are not
proportionally represented in this review(33).
Widespread measles vaccination
increases the average age of measles infection at
the population level by decreasing the force of
infection(34). Data from the US Centers for Disease
Control and the recent WHO measles CFR review
suggest that children <5 yrs infected with measles
have increased mortality in comparison to children
infected at an older age(2,5). Due to the small
number of Indian CFR studies reporting the age of
study participants, we were not able to
statistically test differences between age groups.
Nevertheless, a visual analysis of the Indian data
in Fig. 2 suggests that measles CFR is
decreased in children >5 years.
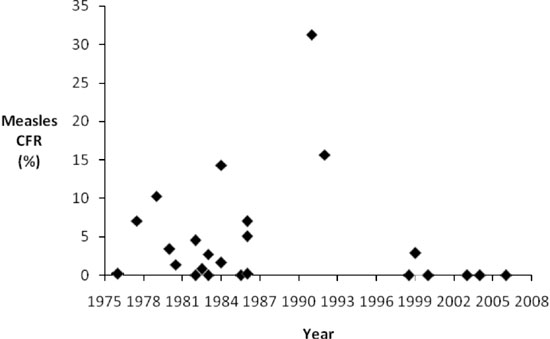 |
|
Fig.2 Measles case
fatality ratio by year of study (Studies with
multiple years of follow-up are plotted at
study midpoint). |
A single dose measles vaccine is
estimated to be 85% efficacious in preventing
measles disease, and as a result a proportion of the
total measles cases occurring in a community are
expected to have been previously vaccinated(35). The
proportion of total measles cases previously
vaccinated in a community is anticipated to increase
as vaccine coverage increases(36). For example, if
measles vaccine coverage for a population is 50%,
13% of the total measles cases are expected to have
been previously vaccinated. Whereas, if vaccination
coverage is 90%, 57% of the total measles cases are
expected to have been previously vaccinated.
Multiple observational studies have found decreased
measles mortality or measles complications in the
previously vacci-nated(2,37-39).There is clear
evidence of partial immunity in some studies, but
confounding by differential access to health care
could be a factor in some studies. When measles
vaccination coverage increases, the expected
proportion of total cases previously vaccinated
increases, and in turn, the population case fatality
ratio likely decreases.
Vitamin A deficiency is a known
risk factor for measles mortality(40). Since 1987,
the WHO and UNICEF have recommended vitamin A
treatment of children with measles(41). A
meta-analysis of randomized controlled trials found
200,000 IU of vitamin A given for 2 days was
associated with a 64% reduction in overall
mortality(42). Neverthe-less, measles case
management with vitamin A may not have considerably
affected Indian CFRs at the population level, since
coverage of vitamin A treatment has been shown to be
low in multiple communities with high levels of
measles transmission. A recent observational study
in Madhya Pradesh found that only 15.8% of measles
cases received therapeutic doses of vitamin A and
another study conducted in slum areas of Kolkata
found only 8.6% were treated(19,32). Routine vitamin
A supplementation is also thought to decrease
measles case fatality; however, the data suggest
supplementation may not be as effective in
preventing measles mortality as vitamin A
administration at the onset of measles(43). Coverage
of vitamin A supplementation may also be low in high
risk populations. In a recent study in the slums of
Delhi, only 37.6% percent of children 12-23 received
a vitamin A supplement(44). Vitamin A treatment and
routine supplementation have likely contributed to
declining CFRs in India, but due to low coverage in
communities at high risk for measles disease and
mortality, the impact on population CFRs may not be
considerable.
The data also suggest higher CFRs
in rural areas compared to urban communities. This
difference may be attributable to differences in
access to health care and vaccination services. No
significant differences were found by study design
or for studies conducted in outbreak vs.
endemic settings. These findings are similar to the
results of the WHO case fatality review(5).
Overall, this review suggests
measles CFR may be declining in India over time. We
theorize that increased measles vaccination coverage
is the main contributor to the decline. The impact
of increasing vaccination coverage on measles
mortality is greater than that expected from
prevention of measles disease alone; since at higher
coverage levels, the average age of infection is
older and a larger proportion of measles cases are
expected to have been previously vaccinated. Vitamin
A treatment and supplementation decrease an
individual’s risk of measles mortality, but the
impact in India at the population level may be
minimal due to low coverage. In order to continue to
decrease measles CFR in India; measles vaccination,
vitamin A treatment, and routine vitamin A
supplementation coverage should be increased. In
addition, the cost effective strategy of introducing
supplementary immunization activities to provide
children with two doses of measles vaccine as well
as increase single dose coverage could also
significantly decrease mortality(45). India has
greatly reduced the total number of measles cases
and deaths over the past few decades, yet much more
needs to be done to decrease the substantial burden
of this preventable disease.
1. Wolfson LJ, Strebel PM,
Gacic-Dobo M, Hoekstra EJ, McFarland JW, Hersh BS.
Has the 2005 measles mortality reduction goal been
achieved? A natural history modelling study. Lancet
2007; 369:191-200.
2. Perry RT, Halsey NA. The
clinical significance of measles: a review. J Infect
Dis 2004;189 Suppl 1:S4-16.
3. Byass P. Measles control in
the 1990s: generic protocol for determining measles
case fatality rates in a community, either during an
epidemic or in a high endemic area. (WHO/EPI/GEN/93.3).
Geneva: WHO; 1993.
4. Narain JP, Banerjee KB.
Measles in India: epidemiology and control. Indian J
Pediatr 1989; 56: 463-472.
5. Wolfson LJ, Grais RF, Luquero
FJ, Birmingham ME, Strebel PM. Estimates of measles
case fatality ratios: a comprehensive review of
community-based studies. Int J Epidemiol 2009; 38:
192-205.
6. Singh J, Sharma RS, Verghese
T. Measles mortality in India: a review of community
based studies. J Commun Dis 1994; 26: 203-214.
7. Kruskal WH, Wallis WA. Use of
ranks in one-criterion variance analysis. J Amer
Statist Assn 1952; 47: 583-621.
8. Dhanoa J, Cowan B. Measles in
the community- a study in non hospitalised young
children in Punjab. J Trop Pediatr 1982; 28: 59-61.
9. Chand P, Rai RN, Chawla U,
Tripathi KC, Datta KK. Epidemiology of measles-a
thirteen years prospective study in a village. J
Commun Dis 1989; 21: 190-199.
10. Garai R, Chakraborty AK.
Measles in a rural community. Indian J Public Health
1981; 24: 150-153.
11. John TJ, Joseph A, George TI,
Radhakrishnan J, Singh RP, George K. Epidemiology
and prevention of measles in rural south India.
Indian J Med Res 1980; 72: 153-158.
12. Cherian T, Joseph A, John TJ.
Low antibody response in infants with measles and
children with subclinical measles virus infection. J
Trop Med Hyg 1984; 87: 27-31.
13. Vasudev JP, Nandan D, Chandra
R, Srivastava BC. Post measles complications in a
rural population. J Commun Dis 1983; 15: 249-252.
14. Swami SS, Chandra S, Dudani
IU, Sharma R, Mathur MM. Epidemiology of measles in
western Rajasthan. J Commun Dis 1987; 19: 370-372.
15. Jajoo UN, Chhabra S, Gupta
OP, Jain AP. Measles epidemic in rural community
near Sevagram. Indian J Public Health 1984; 28:
204-207.
16. Sharma RS, Kaushic VK, Johri
SP, Ray SN. An epidemiological investigation of
measles outbreak in Alwar-Rajasthan. J Commun Dis
1984; 16: 299-303.
17. Lakhanpal U, Rathore MS.
Epidemiology of measles in rural area of Punjab. J
Commun Dis 1986; 18: 185-188.
18. Bhatia R. Measles outbreak in
village Tophema in Nagaland. J Commun Dis 1985; 17:
185-189.
19. Rao RS, Kumari J, Rao TS,
Narashimham VL. Measles in a rural community. J
Commun Dis 1988; 20: 131-135.
20. Lobo J, Reddaiah VP, Kapoor
SK, Nath LM. Epidemiology of measles in a rural
community. Indian J Pediatr 1987; 54: 261-265.
21. Sharma RS. An epidemiological
study of measles epidemic in district Bhilwara,
Rajasthan. J Commun Dis 1988; 20: 301-311.
22. Mangal N, Shah K, Sitaraman
S. Epidemiological study of measles in urban (slum)
area of Jaipur. Indian Pediatr 1990; 27: 1216-1217.
23. Gupta BP, Swami HM, Bhardwaj
AK, Vaidya NK, Sharma CD, Kaushal RK. An outbreak of
measles in a remote tribal area of Himachal Pradesh.
Indian J Comm Health 1989; 5: 25-28.
24. Narain JP, Khare S, Rana SR,
Banerjee KB. Epidemic measles in an isolated
unvaccinated population, India. Int J
Epidemiol 1989; 18: 952-958.
25. Satpathy SK, Chakraborty AK.
Epidemio-logical study of measles in Singur, West
Bengal. J Commun Dis 1990; 22: 23-26.
26. Risbud AR, Prasad SR,
Mehendale SM, Mawar N, Shaikh N, Umrani UB, et
al. Measles outbreak in a tribal population of
Thane district, Maharashtra. Indian Pediatr 1994;
31: 543-551.
27. Thakur JS, Ratho RK, Bhatia
SP, Grover R, Issaivanan M, Ahmed B, et al.
Measles outbreak in a periurban area of Chandigarh:
need for improving vaccine coverage and
strengthening surveillance. Indian J Pediatr 2002;
69: 33-37.
28. John S, Sanghi S, Prasad S,
Bose A, George K. Two doses of measles vaccine: are
some states in India ready for it? J Trop Pediatr
2009; 55: 253-256.
29. Ray SK, Mallik S, Munsi AK,
Mitra SP, Baur B, Kumar S. Epidemiological study of
measles in slum areas of Kolkata. Indian J Pediatr
2004; 71: 583-586.
30. Sharma MK, Bhatia V, Swami
HM. Outbreak of measles amongst vaccinated children
in a slum of Chandigarh. Indian J Med Sci
2004;58: 47-53.
31. Gupta BP, Sharma S. Measles
Outbreak in a rural area near Shimla. Indian J
Community Med. 2006; 31: 106-108.
32. Mishra A, Mishra S, Jain P,
Bhadoriya RS, Mishra R, Lahariya C. Measles related
complications and the role of vitamin A
supplementation. Indian J Pediatr 2008; 75: 887-890.
33. Department of Family Welfare,
Ministry of Health and Family Welfare. Multi Year
Strategic Plan 2005-2010. Universal Immunization
Programme. Government of India; 2005.
34. Grenfell BT, Anderson RM. The
estimation of age-related rates of infection from
case notifications and serological data. J Hyg (Lond)
1985; 95: 419-436.
35. Cutts FT, Grabowsky M,
Markowitz LE. The effect of dose and strain of live
attenuated measles vaccines on serological responses
in young infants. Biologicals 1995; 23: 95-106.
36. Orenstein WA, Bernier RH,
Dondero TJ, Hinman AR, Marks JS, et al. Field
evaluation of vaccine efficacy. Bull World
Health Organ 1985; 63: 1055-1068.
37. Aaby P, Bukh J, Lisse IM, da
Silva MC. Decline in measles mortality: nutrition,
age at infection, or exposure? Br Med J (Clin Res
Ed) 1988; 296: 1225-1228.
38. Byass P, Adedeji MD, Mongdem
JG, Zwandor AC, Brew-Graves SH, Clements CJ.
Assessment and possible control of endemic measles
in urban Nigeria. J Public Health Med 1995;17:
140-145.
39. Hull HF, Williams PJ,
Oldfield F. Measles mortality and vaccine efficacy
in rural West Africa. Lancet 1983;1: 972-75.
40. Sommer A, West KP. Vitamin A
deficiency: health, survival, and vision. New York:
Oxford University Press; 1996.
41. WHO. Joint WHO/UNICEF
statement on vitamin A for measles. International
Nursing Review 1988; 35: 21.
42. D’Souza RM, D’Souza R.
Vitamin A for the treatment of children with
measles–a systematic review. J Trop Pediatr 2002;
48: 323-327.
43. The Vitamin A and Pneumonia
Working Group. Potential interventions for the
prevention of childhood pneumonia in developing
countries: A meta-analysis of data from field trials
to assess the impact of Vitamin A supplementation on
pneumonia morbidity and mortality. Bull WHO
1995; 73: 609-619.
44. Sachdeva S, Datta U. Vitamin
A-first dose supplement coverage evaluation amongst
children aged 12-23 months residing in slums of
Delhi, India. Indian J Ophthalmol 2009; 57: 299-303.
45. Dabral M. Cost effectiveness of supplementary
immunization for measles in India. Indian Pediatr
2009; 46: 957-962.

