|
|
|
Indian Pediatr 2009;46: 975-982 |
 |
Immunogenicity and Safety of a Pentavalent
Diphtheria, Tetanus, Acellular Pertussis, Inactivated
Poliovirus, Haemophilus influenzae Type b Conjugate
Combination
Vaccine (Pentaxim™) with Hepatitis B Vaccine |
|
AK Dutta, VP Verghese*, HK Pemde, LG Mathew* and E Ortiz
…
From Lady Hardinge Medical College and Associated
Hospitals, New Delhi, India;* Christian Medical College Hospital, Vellore,
Tamil Nadu, India; and, …Sanofi
Pasteur, Lyon, France.
Correspondence to: Esteban Ortiz, Global Scientific and
Medical Affairs, Sanofi Pasteur, 2 Avenue Pont Pasteur, 69007, Lyon,
France.
Email: [email protected]
Manuscript received: April 13, 2009;
Initial review: May 12, 2009;
Accepted: September 30, 2009.
ClinicalTrials.gov
identifier: NCT00259337 |
|
Abstract
Objective: To obtain immunogenicity and safety
data for a pentavalent combination vaccine (diphtheria, tetanus,
acellular pertussis, inactivated poliovirus, Hib
polysaccharide-conjugate).
Design: Multicenter, open, Phase III clinical
study. A DTaP-IPV//PRP~T vaccine (PentaximTM) was given at 6,10,14 weeks
of age; and Hepatitis B vaccine at 0,6,14 or at 6,10,14 weeks of age.
Immunogenicity assessed 1 month post-3rd dose; safety assessed for 30
minutes by the investigator, then by parents and investigators to 8 days
and 30 days post-vaccination.
Setting: Tertiary-care hospitals.
Participants/patients: 226 healthy Indian infants
(6 weeks of age).
Main outcome measures: Immunogenicity and safety.
Results: Immunogenicity was high for each vaccine
antigen, and similar to a historical control study (France) following a
2,3,4 month of age administration schedule. Post-3rd dose, 98.6% of
subjects had anti-PRP ³0.15 mg/mL
and 90.0% had titers ³1.0 mg/mL; the
anti-PRP GMT was 4.1 µg/mL. Seroprotection rates for diphtheria and
tetanus (³0.01 IU/mL) were 99.1% and
100%; and 100%,99.1% and 100%, for polio types 1,2 and 3 (³8
[1/dil]) respectively. Anti-polio GMTs were 440.5,458.9, and 1510.7 (1/dil)
for types 1,2 and 3 respectively. The vaccine response rates to
pertussis antigens (4-fold increase in antibody concentration) were
93.7% for PT and 85.7% for FHA; the 2-fold increase was 97.1% and 92.4%.
Vaccine reactogenicity was low with adverse reaction incidence not
increasing with subsequent doses.
Conclusion: The DTaP-IPV//PRP~T vaccine, given
concomitantly with monovalent hepatitis B vaccine, was highly
immunogenic at 6, 10 and 14 weeks of age in infants in India. The
vaccine was well tolerated.
Keywords: pentavalent combined vaccine, primary series, EPI
schedule, immunogenicity, safety.
|
|
V
accines combining whole cell
Bordetella pertussis (wP) antigens with diphtheria (D) and tetanus (T)
toxoids (DTwP) have been central to the WHO expanded program of
immunization (EPI). The valences included in modern combination vaccines
reflect current EPI recommendations. In India, DTwP has been a part of the
Universal Immunization Program (UIP) since 1985, and the Indian Academy of
Pediatrics (IAP) recommends Hib vaccination and inactivated polio vaccine
(IPV) for all children who can afford it after one-to-one discussion with
parents(1). The use of combination vaccines reduces the number of required
injections, the number of clinic visits and the discomfort for children. A
number of DTwP-based combination vaccines containing Hib have recently
been evaluated in India(2).
In the context of the World Health Organisation (WHO)
recommendation to cease OPV use in the post-eradication era to minimise
the impact of circulating vaccine-derived polioviruses (cVDPVs) and
vaccine-associated paralytic poliomyelitis (VAPP), the shift towards the
use of IPV is vital(3,4), and its incorporation in combination vaccines is
an important step.
Acellular pertussis (aP) vaccines containing purified
B. pertussis antigens are better tolerated than wP vaccines(5).
Combination vaccines incorporating an aP component have become widely
adopted over the last 10 years, and are now included in the national
immunization programs in North America, most western European countries,
some Asian countries, Mexico, Turkey and South Africa(6).
The WHO position on pertussis vaccines is that ‘‘the
best aP vaccines have shown similar protective efficacy as the best wP
vaccines, and that all licensed vaccines have proved to be highly
effective in controlling pertussis in infants and young children’’(7). In
addition, the WHO position paper on aP vaccines states that ‘‘although
most efficacy and effectiveness studies on aP vaccines have been conducted
in industrialized countries, the new DTaP vaccines are expected to be
efficacious in all regions of the world.’’
Various aP combination vaccines have been developed,
including a liquid DTaP-IPV combination used to reconstitute a lyophilized
Hib conjugate vaccine (PRP~T) at the time of injection, which was used in
the present study. Each antigen is well-known as a stand-alone vaccine;
the PRP~T and IPV vaccines are licensed worldwide including in India and
both are WHO pre-qualified(8). The DTaP-IPV//PRP~T vaccine has been
licensed since 1997 as Pentaxim™/Pentavac™ in >85 countries worldwide,
including India in 2007. The exclusion of a hepatitis B (Hep B) component
from this pentavalent vaccine affords greater flexibility in the
administration schedule of the Hep B vaccine.
The present study was carried out to obtain
immunogenicity, reactogenicity and safety data in Indian subjects
following administration of this DTaP-IPV//PRP~T pentavalent vaccine in
the EPI immunization schedule (6,10,14 weeks of age) with a separate Hep B
vaccine being administered at either 0,6,14 or 6,10,14 weeks of age,
according to the IAP recommendations for immunization in India.
Methods
Study Design
This prospective, non-comparative, Phase III, open
clinical study was performed at two medical centers in India - Lady
Hardinge Medical College and Associated Hospitals in New Delhi and
Christian Medical College Hospital, Vellore, Tamil Nadu. The study
protocol and consent form were approved by the relevant institutional
review boards before study initiation and the study conformed to local
regulations, GCP and applicable ICH guidelines, and the ethical principles
of the Declaration of Helsinki. Written informed consent was obtained from
a parent/legal guardian of each subject before enrolment.
Subjects
Healthy fullterm ( ³37
weeks) infants weighing
³2.5
kg at birth were eligible. Each subject received the DTaP-IPV//PRP~T study
vaccine (6,10,14 weeks of age) and hepatitis B (Hep B) vaccine (0,6,14 or
at 6,10,14 weeks of age) according to the Indian National Immunization
schedule and the clinical practice in each study center. A physical
examination and medical review were performed before enrolment, and the
inclusion and exclusion criteria were verified. Exclusion criteria
included congenital or acquired immunodeficiency; immunosuppressive
therapy; systemic hypersensitivity to any vaccine component; chronic
illness that could interfere with trial conduct/completion; previous
administration of blood or blood-derived products; any vaccination
preceding the trial vaccination (except Bacille Calmette-Guérin [BCG] and
hepatitis B vaccination); history of, or vaccination against, diphtheria,
tetanus, pertussis, poliomyelitis, Hep B or Hib; thrombocytopenia/bleeding
disorder contraindicating intramuscular vaccination; and, history of
seizures.
Vaccines
The pentavalent vaccine (batch Z2044-1, Pentaxim™) was
produced and supplied by Sanofi Pasteur, France, and stored between 2ºC
and 8ºC. Each 0.5 mL dose of DTaP-IPV//PRP~T contained
³30
IU (25Lf) of diphtheria toxoid,
³40
IU (10Lf) of tetanus toxoid, 25 µg of pertussis toxoid (PT), 25 µg of
filamentous hemagglutinin (FHA), 40 D antigen units (DU) of poliovirus
type 1 (Mahoney), 8 DU of poliovirus type 2 (MEF-1), 32 DU of poliovirus
type 3 (Saukett), and 10 µg polyribosyl-ribitol-phosphate (PRP) Hib
capsule polysaccharide conjugated to tetanus protein. The lyophilized
PRP~T component was reconstituted with the liquid DTaP-IPV vaccine
immediately before vaccination. The recombinant Hep B vaccine (Euvax B™,
LG Life Sciences, Iksan, Korea, batch UVA05005), contained 10 µg of
recombinant HBsAg; this vaccine is licensed and commercially available in
India. The pentavalent vaccine and the hepatitis B vaccine were
administered by intramuscular injection into the right and left anterior
thigh, respectively.
Serology
Blood samples (4 mL) were collected just before the
first vaccine dose at 6 weeks of age, and at 18 weeks of age,
approximately 1 month after the third vaccination, and serologic analyses
were performed at the Sanofi Pasteur central laboratory in Swiftwater,
Pennsylvania, USA. Anti-HBs antibody titer determinations were not
performed as it was not possible to prospectively plan the proportion of
subjects who would receive the hepatitis B vaccination at 0,6,14 weeks or
at 6,10,14 weeks of age, due to different Hep B vaccination schedules
between study centers.
Reactogenicity and Safety
Safety data were only collected for the pentavalent
vaccine. Following an initial assessment by the Investigator of immediate
adverse events occurring in the 30 minutes after each vaccination,
parents/legal guardians recorded solicited injection site reactions
(redness, swelling and tenderness) and solicited systemic reactions (fever
[axillary temperature
³37.4ºC], vomiting, abnormal crying,
drowsiness, loss of appetite and irritability) on diary cards daily for 8
days after each vaccination. Unsolicited injection site and systemic
reactions (with onset date, resolution, and intensity) were recorded for
30 days after each vaccination. Serious adverse events (SAEs) were
reported throughout the conduct of the study. Solicited and unsolicited
local and systemic adverse events were graded according to the scales
described below as mild, moderate, or severe. Mild, moderate or severe
tenderness were defined as ‘minor reaction when injection site is
touched’, ‘cries and protests when injection site is touched’, and ‘cries
when injected limb is moved, or the movement of the injected limb is
reduced’. For erythema and swelling, a diameter of <2.5 cm was graded as
mild, 2.5 to 5 cm as moderate and
³5
cm as severe. Mild, moderate and severe fever were defined as axillary
temperature ³37.4ºC
to 37.9ºC, ³38ºC
to 38.9ºC, and ³39ºC,
respectively.
Statistical Analysis
Seroprotection and vaccine response rates were
calculated with their corresponding 95% confidence intervals (CI) using
the exact binomial method. Pre-defined seroprotection levels were: anti-PRP
³0.15
and ³1.0
µg/mL; anti-polio ³8
(1/dil); anti-diphtheria and anti-tetanus
³0.01
IU/mL. The pertussis antigens were assessed using
³4-fold
and ³2-fold
increases in antibody concentration from pre- to post-vaccination state.
Geometric mean titers (GMTs) were calculated with 95%
CIs using the normal approximation. Reverse Cumulative Distribution Curves
(RCDCs) for pre- and post-vaccination antibody titers were also derived.
The statistical analysis was descriptive; no hypothesis was tested.
The sample size calculation of 226 subjects allowed an
indirect, descriptive comparison with a historical control study conducted
in France using the same combined vaccine but given at 2, 3 and 4 months
of age(26). A drop-out rate of 20% was assumed in order to ensure at least
180 evaluable subjects.
Results
Study population
A total of 226 infants were enrolled. There were
slightly more male (53.1%) than female (46.9%) infants. The mean age
(±standard deviation) when the first vaccine dose was administered was
45.0±2.9 days (about 6.4 weeks) and the mean weight was 4.5±0.5 kg. Ten
subjects did not complete the study; six were withdrawn by parents (one
due to migration away from the area and five due to personal reasons not
related to an adverse event), two were withdrawn because of protocol
violations (receipt of oral polio vaccine during the study), and two were
lost to follow up. There were no withdrawals because of adverse events.
Immunogenicity
The seroprotection and vaccine response rates for the
study vaccine and historical control study are summarized in Table
I. In addition to the criteria presented in Table 1,
90.0% of subjects had anti-PRP
³1.0
µg/mL after the third dose, and 97.1% and 92.4% of subjects had
³2-fold
increases in antibody titer for PT and FHA. The tetanus seroprotection
rate was very high before the first injection, with 99.5% of subjects
having anti-tetanus antibody titers
³0.01
IU/mL and 97.2% having titers
³0.1
IU/mL. GMTs increased strongly following the primary vaccination (Table
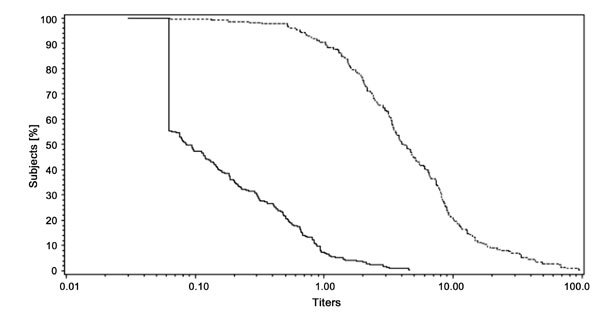
II). Fig.1 shows a strong, linear increase in antibody
titers for anti-PRP antibodies (µg/mL); a similar effect was seen for the
remaining antigens.
Table I
Seroprotection/Vaccine Response Rates to DTaP-IPV/PRP~T Pentavalent Vaccine
|
|
Historical control* |
Study vaccine† |
|
|
Rate % (95% CI) |
Rate % (95% CI) |
|
Anti-diphtheria ≥0.01 IU/mL |
100 (95.9;100) |
99.1 (96.6;99.9) |
|
Anti-tetanus ≥0.01 IU/mL |
100 (95.9;100) |
100 (98.3;100) |
|
Anti-polio 1 ≥8 (1/dil) |
97.0 (91.5;99.4) |
100 (98.3;100) |
|
Anti-polio 2 ≥8 (1/dil) |
100 (96.4;100) |
99.1 (96.6;99.9) |
|
Anti-polio 3 ≥8 (1/dil) |
99.0 (94.6;100) |
100 (98.3;100) |
|
Anti-PRP ≥0.15 µg/mL |
98.0 (93.0;99.8) |
98.6 (95.9;99.7) |
|
Anti-PT ≥4-fold increase EU/mL |
89.6 (81.7;94.9) |
93.7 (89.5;96.6) |
|
Anti-FHA ≥4-fold increase EU/mL |
89.5 (81.5;94.8) |
85.7 (80.2;90.1) |
|
* DTaP-IPV//PRP~T pentavalent vaccine at 2, 3
and 4 months of age (French historical control study - Mallet et al,
1997[8]); † DTaP-IPV//PRP~T pentavalent vaccine at 6, 10 and 14 weeks
of age. Anti-PRP measured by Farr-type radioimmunoassay (RIA) in
comparison to an American Food and Drug Administration (FDA) human
reference serum, lower limit of quantification (LLOQ) 0.06 µg/mL;
anti-FHA and anti-PT measured by ELISA in comparison to sanofi pasteur
reference standards, LLOQ 2 EU/mL; anti-tetanus measured by ELISA in
comparison to a WHO reference standard, LLOQ 0.01 IU/mL; anti-polio
were titrated by microneutralization following a modified WHO
standardized procedure using Vero cells and wild-type poliovisuses,
LLOQ 4 (1/dil); anti-diphtheria were titrated using a micrometabolic
inhibition test against a WHO reference standard, LLOQ of 0.005 IU/mL. |
Table II
Geometric Mean Titers (GMTs) Before the First Dose and One Month After the Third
Dose of the Study Vaccine
| |
Pre-first dose |
Post-priming* |
| |
GMT (95% CI) |
GMT (95% CI) |
|
Anti-diphtheria (IU/mL) |
0.028 (0.022; 0.033) |
0.046 (0.040; 0.053) |
|
Anti-tetanus (IU/mL) |
1.96 (1.69; 2.28) |
0.93 (0.86; 1.0) |
|
Anti-polio 1 (1/dil) |
18.1 (15.1; 21.5) |
440.5 (363.4; 533.9) |
|
Anti-polio 2 (1/dil) |
20.4 (16.6; 25.2) |
458.9 (361.4; 582.6) |
|
Anti-polio 3 (1/dil) |
9.9 (8.6; 11.5) |
1510.7 (1283.9; 1777.6) |
| Anti-PRP
(µg/mL) |
0.11 (0.09; 0.14) |
4.17 (3.52; 4.93) |
| Anti-PT (EU/mL) |
4.9 (4.0; 5.9) |
321.1 (294.0; 350.8) |
| Anti-FHA
(EU/mL) |
5.1 (4.3; 5.9) |
97.6 (94.6; 99.2) |
|
* DTaP-IPV//PRP~T pentavalent
vaccine at 6, 10 and 14 weeks of age. |
 |
|
Fig.1 RCDCs for anti-PRP antibody titers
before (6 weeks of age) and 1 month after primary vaccination (18
weeks of age). |
Safety and Reactogenicity
Solicited symptoms after any dose and after each dose
are summarized in Table III. Injection site tenderness was
the most common symptom, but was severe only after two injections (0.3% of
doses). The incidence of erythema and swelling was also low. All solicited
injection site reactions occurred within 3 days after vaccination except
one case of erythema/redness that occurred between Days 4 and 7. The
percentage of subjects with a specific adverse reaction did not increase
with subsequent doses. Fever was the most frequent systemic reaction, with
at least one episode being reported by 33.0% of subjects (15.3% of doses),
followed by irritability and drowsiness. Only two subjects (0.9%) had
severe fever ( ³39.0ºC
axillary temperature) and only one case of drowsiness was severe.
Table III
Solicited Local Adverse Reactions And Systemic Reactions That Occurred Within 8 Days (Days 0-7)
After Each Dose and After Any Dose of Pentavalent Vaccine
|
|
|
DTaP-IPV//PRP~T vaccine |
|
|
|
Dose 1 |
Dose 2 |
Dose 3 |
Any dose |
|
|
|
n = 224 |
n = 217 |
n = 217 |
n = 658 |
|
|
|
% of doses |
% of doses |
% of doses |
% of doses |
| Local reactions |
|
Tenderness |
Any |
21.9 |
15.7 |
14.3 |
17.3 |
|
|
Severe |
0.4 |
0.5 |
0.0 |
0.3 |
|
Redness |
Any |
9.4 |
5.1 |
3.7 |
6.1 |
|
|
Severe |
0.0 |
0.0 |
0.0 |
0.0 |
|
Swelling |
Any |
6.3 |
3.7 |
5.1 |
5.0 |
|
|
Severe |
0.4 |
0.0 |
0.0 |
0.2 |
| Systemic events |
|
Fever |
Any |
17.0 |
12.4 |
16.6 |
15.3 |
|
|
Severe |
0.4 |
0.5 |
0.0 |
0.3 |
|
Vomiting |
Any |
17.0 |
11.1 |
11.5 |
13.2 |
|
|
Severe |
0.4 |
0.0 |
0.0 |
0.2 |
|
Abnormal crying |
Any |
14.3 |
11.1 |
11.5 |
12.3 |
|
|
Severe |
0.4 |
0.0 |
0.9 |
0.2 |
|
Drowsiness |
Any |
16.5 |
6.9 |
6.9 |
10.2 |
|
|
Severe |
0.4 |
0.0 |
0.0 |
0.2 |
|
Appetite loss |
Any |
11.6 |
7.4 |
7.8 |
9.0 |
|
|
Severe |
0.0 |
0.5 |
0.0 |
0.2 |
|
Irritability |
Any |
15.6 |
12.0 |
13.4 |
13.7 |
|
|
Severe |
0.0 |
0.0 |
0.0 |
0.0 |
Overall, 119 subjects (53.1%) reported at least one
unsolicited AE following vaccine administration. Only one unsolicited AE,
a mild macular rash occurring one day after the first injection and
lasting for 7 days was assessed by the investigator as related to the
vaccination. Eleven subjects experienced at least one SAE; none was
considered to be related to the vaccination and most were diagnoses
commonly observed in infancy such as bronchopneumonia, bronchiolitis, and
gastroenteritis. Four cases of chikungunya or probable chikungunya
occurred. One case of seizure/convulsion was reported 22 days after the
third injection. No hypotonic hyporesponsive episode (HHE) was reported.
All subjects with an SAE recovered, and no subject was withdrawn due to an
adverse event.
Discussion
This clinical study evaluated the immunogenicity and
safety of a DTaP-IPV//PRP~T combination vaccine (Pentaxim™) for primary
immunization at 6,10,14 weeks of age, with a monovalent recombinant
hepatitis B vaccine (Euvax B TM)
given at either 6,10,14 weeks of age or 0,6,14 weeks of age. No control
group wP was included since wP vaccines are routinely available in India,
meaning that participation in such an arm of the study would have offered
no benefit to the infant. Instead, the study design included a descriptive
comparison to a historical control study group given the same vaccine at
2,3,4 months of age in France, where this vaccine has been routinely
used(9). At the time of the study, no historical clinical data on the
immunogenicity of this vaccine given at 6,10,14 weeks of age were
available.
Seroprotection and vaccine response rates were similar
to the control study and also to those subsequently seen in a later study
evaluating the same vaccine for primary vaccination in infants in the
Philippines following the 6,10,14 weeks of age EPI schedule(10). The
statistical comparison was performed using the French data rather than the
Philippine data since at the time of the design and set-up of the study
reported in this article, the Philippine data were not available.
The good seroprotection and GMT response to the IPV
antigens are of particular interest because of the vaccination schedule.
During the study, no subject that was analysed for immunogenicity received
OPV, although the possibility of an effect of herd immunity is
acknowledged since OPV is used routinely in India. However, this putative
herd immunity is difficult to quantify, and a similarly strong response to
the same inactivated polio antigens has been observed in countries where
OPV is no longer used (e.g France, Sweden) (9,11). As such, we do
not consider the polio response to be markedly augmented due to routine
local OPV use. Overall, these results are consistent with the IPV
immunogenicity seen for this vaccine in studies in various European
countries, Chile, and Turkey following vaccination schedules of 2,3,4
months of age, 2,4,6 months of age, and 3,5,12 months of age(11,12,13).
The strong IPV response is particularly relevant in the context of OPV
cessation in the post-eradication era and the planned switch to routine
IPV use.
As there are no recognized serological correlates of
protection for pertussis, 4-fold increases from pre- to post-vaccination
were used to evaluate the anti-PT and FHA response. These data and the
strong increase in GMTs accord with anti-PT and anti-FHA responses to this
2-component acellular pertussis vaccine reported in 36 clinical trials
conducted in 17 countries in Europe, North and South America, Africa and
Asia that have included nearly 10,000 subjects(14). The long-term
effectiveness of the pentavalent vaccine on pertussis incidence has been
documented over the past 10 years by the National Surveillance Program in
Sweden using a 3, 5, 12 month schedule(15,16). Although the schedule in
Sweden differs from that in India, we believe that the Swedish
surveillance data are applicable to the EPI administration schedule since
high immunogenicity has been demonstrated from a range of primary series
schedules(11-13). These data have shown that routine primary vaccination
with aP vaccines, including the present pentavalent vaccine (Pentaxim TM),
has resulted in a marked decrease in the incidence of pertussis cases
after the second and third doses and that protection remains high after
the third dose for 8 to 9 years(17). In addition, the WHO position paper
on aP vaccines(7) states that ‘‘although most efficacy and effectiveness
studies on aP vaccines have been conducted in industrialized countries,
the new DTaP vaccines are expected to be efficacious in all regions of the
world.’’
The anti-PRP antibody response is consistent with both
the Philippine study and the control study. Furthermore, this anti-PRP
response is similar to that observed in previous studies in India
conducted with ActHib given either alone, concomitantly or combined with
DTwP vaccines(18,19), highlighting the strong immune response to the Hib
component of the pentavalent vaccine.
The relatively high incidence of minor adverse events
and occasional SAEs associated with wP vaccines has prompted the
development of aP vaccines, and the safety results of this study reflect
the good reactogenicity documented for all aP-based combination
vaccines(20,21).
In summary, the DTaP-IPV//PRP~T pentavalent vaccine was
highly immunogenic for all antigens and well tolerated when administered
in the 6,10,14 weeks of age EPI schedule, consistent with previous data.
The inclusion of IPV makes it easier to incorporate this vaccine
successfully into the UIP in India, in the context of the future cessation
of OPV in the post-eradication era.
Acknowledgments
The authors would like to thank the participating
clinicians at each study site and Clement Weinberger (Le Stylo
Communications) for assistance with the draft manuscript preparation. The
authors would like to acknowledge Fabrice Guitton and Ranjeet Kaur for
study monitoring, Roy Fernando for data management, Valérie Bosch-Castells
for statistical analysis, and Andrew Lane for additional assistance in
finalising the manuscript. FG, RK, RF, VB and AL are employees of Sanofi
Pasteur.
Contributors: AKD, VPV, HKP and LGM were
responsible for trial conduct, data acquisition, data interpretation,
manuscript review and approval. EO was responsible for study design, data
interpretation, manuscript review and approval.
Funding: Sanofi Pasteur, Lyon, France.
Competing interest: EO is an employee of Sanofi
Pasteur which manufactures Pentaxim TM.
|
What is Already Known?
• Pentavalent (DTaP-IPV-Hib) vaccine (Pentaxim™)
is safe and immunogenic in over 85 countries.
What this Study Adds?
• Additional immunogenicity and safety data of
Pentaxim™ following a primary vaccination EPI (6, 10, 14 weeks)
schedule in India, in particular a strong immune response to three
polio antigens. |
References
1. Indian Academy of Pediatrics Committee on
Immunization (IAPCOI). Consensus recommen-dations on immunization, 2008.
Indian Pediatr 2008; 45: 635-648.
2. Shah R, Raghu MB, Shivananda A, Mangayar-karashi S,
Rao I, Rao R, et al. Immunogenicity and safety of an indigenously
developd DTPw-hepatitis B combination vaccine in Indian infants. Indian
Pediatr 2008; 45: 819-823.
3. Polio Eradication Committee, Indian Academy of
Pediatrics, Vashishtha VM, John TJ, Agarwal RK, Kalra A. Universal
immunization program and polio eradication in India. Indian Pediatr 2008;
45: 807-813.
4. Mittal SK, Mathew JL. IPV revisited...yet again.
Indian Pediatr 2008; 45: 390-395.
5. Edwards KM, Decker M. Pertussis vaccines. In:
Plotkin SA, Orenstein WA, editors. Vaccines. 5th ed. Philadelphia PA:
Saunders Co.; 2008. p. 467-518.
6. WHO. Vaccine preventable diseases monitoring system:
Country profiles. Available from: URL: http://www.who.int/vaccines/globalsummary/immunization/countryprofileselect.cfm.
Accessed January 15, 2009.
7. WHO Position Paper. Pertussis vaccines. Weekly
Epidemiol Rec 2005; 80: 29-40.
8. WHO. United Nations prequalified vaccines (WHO list
of vaccines for purchase by UN agencies as of August 2008). Available
from: URL: http://www.who.int/immunization_ standardsn/vaccine_quality/pq_suppliers/en/index.html.
Accessed October 29, 2008.
9. Mallet E, Hoffenbach A, Salomon H, Blondeau C,
Fritzell B. Primary immunization with combined, acellular DTaP-IPV-Act-HIB
vaccine given at 2-3-4 or 2-4-6 months of age. The 15th Annual Meeting of
the European Society for Paediatric Infectious Diseases (ESPID), Paris,
France 1997.
10. Capeding RM, Cadorna-Carlos J, Book-Montellano M,
Ortiz E. Immunogenicity and safety of a DTaP-IPV//PRP~T combination
vaccine at 6, 10,14 weeks of age (EPI schedule) and concomitant Hepatitis
B vaccination at birth, 6, 14 or 6, 10, 14 weeks of age. WHO Bulletin
2008; 86: 443-451.
11. Carlsson RM, Claesson BA, Selstam U, Fagerlund E,
Granstrom M, Blondeau C, et al. Safety and immunogenicity of a
combined diphtheria, tetanus, acellular pertussis-inactivated polio
vaccine- Haemophilus influenzae type b vaccine administered at 2-4-6-13 or
3-5-12 months of age. Pediatr Infect Dis J 1998; 17: 1026-1033.
12. Lagos R, Kotloff, KL Hoffenbach A, San Martin O,
Abrego P, Ureta AM, et al. Clinical acceptability and
immunogenicity of a pentavalent parenteral combination vaccine containing
diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis and
Haemophilus influenzae type b conjugate antigens in two-, four- and six
month-old Chilean infants. Pediatr Infect Dis J 1998; 17: 294-304.
13. Kanra G, Selier T, Yurdakök K, Yavuz T, Baskan S,
Ulukol B, et al. Immunogencity study of a combined diphtheria,
tetanus, acellular pertussis, inactivated poliomyelitis vaccine used to
reconsti-tute a freeze-dried Haemophilus influenzae type b vaccine (DTacP-IPV//PRP-T)
administered simul-taneously with a hepatitis B vaccine at two, three and
four months of life. Vaccine 2000; 18: 947-954.
14. Vidor E, Plotkin SA. Immunogenicity of a
two-component (PT & FHA) acellular pertussis vaccine in various
combinations. Human Vaccines 2008; 4: 328-340.
15. Swedish Institute for Infectious Disease Control.
Pertussis surveillance in Sweden with enhanced follow-up of cohorts
immunized with acellular pertussis vaccines 2007 Appendix 2 SP-MSD.
Available from: URL: http://www.smittsky ddsinstitutet.se/upload/10-y%20report-app%202-%20SP%20MSD.pdf.
Accessed December 4, 2008.
16. Olin P, Hallander HO. Marked decline in pertussis
followed reintroduction of pertussis vaccination in Sweden. Euro Surveill
1999; 4:128-129.
17. Gustafsson L, Hessel L, Storsaeter J, Olin P.
Long-term follow-up of Swedish children vaccinated with acellular
pertussis vaccines at 3, 5, and 12 months of age indicates the need for a
booster dose at 5 to 7 years of age. Pediatr 2006; 118: 978-984.
18. Cherian T, Thomas N, Raghupathy P, Durot I, Dutta
A. Safety and immunogenicity of Haemo-philus influenzae type B
vaccine given in combi-nation with DTwP at 6, 10 and 14 weeks of age.
Indian Pediatr 2002; 39: 427-436.
19. Acharya D, Bhave S, Joshi V, Bavdekar A, Pandit A.
Haemophilus influenzae type b vaccine in India: need and timing,
immunogenecity and tolerance. Indian Pediatr 1997; 34: 9-15.
20. Greco D, Salmaso S, Mastrantonio P, Giuliano M,
Tozzi AE, Anemona A, et al. A controlled trial of two acellular
vaccines and one whole-cell vaccine against pertussis. N Engl J Med 1996;
334: 341-348.
21. Olin P, Rasmussen F, Gustafsson L, Hallander H,
Heijbel H. Randomised controlled trial of two-component, three component,
and five component acellular pertussis vaccines compared with whole-cell
pertussis vaccine. Lancet 1997; 350: 1569-1577.
|
|
|
 |
|

