M. Kaushal, S. Narayan, R. Aggarwal, A. Kapil and
A.K. Deorari
From the Division of Neonatology, Department of
Pediatrics, All India Institute of Medical Sciences, Ansari Nagar, New
Delhi 110 029, India.
Correspondence to: Dr. Rajiv Aggarwal, Assistant
Professor, Department of Pediatrics, All India Institute of Medical
Sciences, Ansari Nagar, New Delhi 110 029, India.
E-mail: [email protected]
Manuscript received: June 20, 2003, Initial review
completed: October 15, 2003;
Revision accepted: February 24, 2004.
An experiment was conducted to evaluate the
efficacy of epidural bacterial filters (pore size 22nm) in the
filtration of pathogenic bacteria from clear intravenous (IV)
fluids. Fifty mL of sterile dextrose was mixed with a known
quantity of bacteria and infused through an epidural bacterial
filter by a syringe pump at a rate of 2 ml per hour for 24 hours.
Cultures were done from the remaining fluid in the syringe, the
filtrate in the urobag, as well as the bacterial solution at the
end of 24 hours. The efficacy of the filter against bolus doses
was also evaluated. Staphylococcus aureus and Pseudomonas (80cc
strain) were chosen. Initial runs conducted without the filters
showed that bacteria could be isolated from the syringe and the
urobag at the end of 24 hours. The filtrate in all runs and that
tested for efficacy against IV bolus dose was sterile. However,
four filters in the experiment using bolus doses, got clogged and
had to be discarded. The epidural bacterial filter proved 100%
effective in removing pathogenic bacteria from clear IV fluids
under experimental conditions.
Key words: Bacterial infections, Epidural filters.
Intravenous (IV) fluids form the mainstay of
treatment in a large number of very low birth weight (<1500 grams)
and sick neonates. These neonates are prone to infection and IV
lines act as an easy portal of entry for pathogens. Sepsis is a
major cause of morbidity and mortality in neonates and contamination
of IV fluids during use in the hospital continues to be of concern.
This can be prevented with the use of intravenous filters(l,2).
There are though few studies which have shown no extra benefit where
laminar flow is used(3). The other benefit of IV filters is
decreased phlebitis(4). A number of on -line antibacterial filters
are available in the market. However their prohibitive cost limits
their widespread use. The cost of available IV antibacterial filters
is approximately Rs. 400. They last for 24 to 72 hrs. There are some
cheaper alternatives like the epidural filters. The cost of the
epidural filter is approximately Rs. 100. The limitation with this
alternative is that it has not been studied for use with syringe
infusion pumps. Rather, they have been tested in gravity dependent
flow systems. This experimental study was designed to evaluate
efficacy of an epidural antibacterial filter for use as an IV filter
for clear fluids administered by a syringe pump and contaminated
with known pathogen.
Subjects and Methods
The experimental study was done in the
microbiological laboratory under strict quality control. Sterile 10%
dextrose was used as the infusing fluid. The efficacy of the
nitrocellulose epidural filter (0.22µm pore size) made by Vygon was
tested for known pathogenic strains in the laboratory. The strains
tested were Staphylococcus aureus ATCC number 80 cc size l µm
and Pseudomonas aeruginosa A TCC number 88 cc size 0.5 µm.
The experiment was conducted in multiple runs.
Experiment
Sterile syringes (50 mL each) were loaded with 50
mL of 10% dextrose and spiked with a bacterial suspension equivalent
to 106/mL of bacteria. Each syringe was connected to a sterile IV
infusion line. To the distal end of this IV line, an antibacterial
filter was attached, and the filtrate was collected into a sterile
urobag. The entire system was thus a sterile one. All connections
and manipulations were done in the same manner under strict aseptic
conditions. Syringe pump was used to infuse the fluid in each
syringe at 2 mL/hr for 24 hrs.
Preparation of study inoculum
Plates were inoculated with Staphylo-coccus
aureus ATCC No. 80 cc and Pseudomonas aeruginosa ATCC No.
88 cc strain and incubated at 37ºC for 24 hours. Isolated single
colony was sub cultured in 10% dextrose and incubated at 37ºC
overnight. The inoculum preparation was adjusted to 0.5 Nacfarland’s
standard to obtain a total cell concentration of 106 cells/mL of 10%
dextrose infusion.
Infusion without the filter: (control)
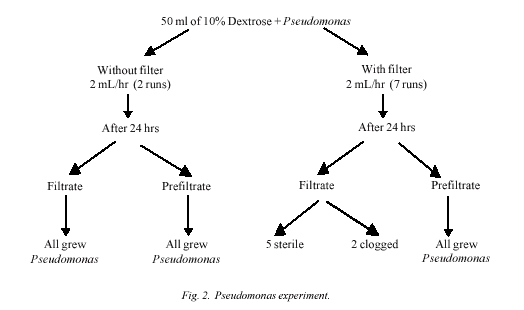
Long-term viability of the inoculum was monitored
by the initial two runs, which acted as control (Figs. 1 and 2)
syringes containing either of the two organisms. The spiked IV fluid
with each organism and without the filter was infused at 2 mL/hr and
the elute was collected after 24 hrs and cultured. This was to
ensure the viability of bacteria in the sterile urobag at the end of
24 hours of running the infusion.
Fig.1 Staph. aureus experiment.
Infusion with the filter: (study)
Subsequently six experiments with
Staphylococcus aureus and seven experiments with Pseudomonas
aerugenosa were run to collect filtrate in urobag with online
bacterial filter. At the end of 24 hrs, of each experiment filtrate
as well as prefiltrate elute were cultured for bacterial growth on
nutrient agar.
Bolus doses
To evaluate the efficacy of the filter against
fast bolus infusions administered at higher rates, three filters
were tested for each bacteria. Two mL of inoculum of 106/mL in 10%
dextrose of S. aureus and P. aerugenosa were loaded
separately under same sterile condition. Six boluses were infused
through each filter at an interval of 5 min. The prefiltrate and
post filtrate elute were cultured on nutrient agar.
Results
Control infusion
The initial experiments, which acted as control
without the filters, showed both the bacteria to be recoverable at
the end of 24 hrs from the syringe and the urobag. A total of
thirteen experiments using filter were conducted. Out of the
thirteen experiments, the inoculum was Pseudomonas in seven
and Staphylococcus aureus in six experiments.
Infusion study
Three experiments had to be abandoned. Out of
these three, the inoculum was S. aureus in one and
Pseudomonas in two experiments. Two of these had to be abandoned
because of a malfunctioning of syringe pump and one because filter
got clogged. The filter, which got clogged had Pseudomonas as
inoculum. In the remaining five experiments for each of the
organism, despite the bacteria being isolated from the prefiltrate,
the filtrate was sterile at the end of 24 hours.
Bolus study
Nine filters were tested for efficacy of filter
to withstand IV bolus doses. In the three bacterial filters in which
S. aureus inoculums had been pushed six times as bolus doses
of 2 mL each were found to have all six sterile filtrate while from
pre filtrate organism could be cultured. Three filters in which
Pseudomonas inoculums were pushed got clogged. The experiment
was repeated with three more filters using Pseudomonas as
inoculum. Out of the three filters, in two filters after pushing six
times 2 mL each as bolus doses, it was seen that all the six
filtrates were sterile and prefiltrate grew organism, but one filter
again got clogged. The study was again repeated with three more
filters using Pseudomonas as inoculum after the inoculum was
checked for the load. All the three filters had sterile filtrate
after each push repeated six times the reason for clogging in the
previous filters was probably too much of inoculum load.
Discussion
The epidural bacterial filters proved 100%
effective under experimental conditions in filtering out
Staphylococcus aureus and Pseudomonas when used with
syringe pump. Also, it was 100% effective in filtering the same
bacteriae when pushed through the filter six times each. The
experiments in which the filter got clogged could be explained by
the heavy inoculum of Pseudomonas aeruginosa, which may not
be simulated in real life situation. An epidural bacterial filter
(pore size being 22 nm) costs about 50% of other available bacterial
filters. The cost difference is Rs. 300. This may be useful
especially in situations where IV fluid is to be given for shorter
duration where they may be more cost effective. On the other hand,
costly filters with efficacy for 72 hrs are not of any added
advantage. It is very important in develop- ing countries like ours
to have such cost effective and cheaper alternatives. Controlled
clinical trials would be needed to deter- mine the effectiveness and
clinical efficacy of this low cost, readily available epidural
filter.
Conclusion
The epidural filter was found to be effective in
experimental conditions in filtering out gram positive (Staph.
aureus) and gram negative (Pseudomonas 80 cc strain)
organisms when infused at the rate of 2 mL/hr for 24 hours using a
syringe pump and using 5% dextrose as the parenteral solution. It
was also seen that the filter was able to withstand the bolus doses,
which were much faster than the infusion rates in keeping away the
two above mentioned laboratory strains. Under experimental
conditions two out of nine filters were clogged due to inoculum
load. There is scope for clinical trials using these low cost
epidural filters in preventing nosocomial infections.
Acknowledgement
The authors are thankful to Vygon Company for
providing filters for the experiment.
Contributors: The study was designed by AKD.
MK and SN were responsible for conducting the experiment under the
supervision of the Micro-biologist AKMK and RA were responsible for
preparing the manuscript. RA would be responsible for any
correspondence related to this manuscript.
Funding: The epidural filters were provided
by the Vygon company.
Competing interests: None.
