|
|
|
Indian Pediatr 2021;58: 424-429 |
 |
Reference Ranges of Different Lymphocyte
Subsets in Indian Children: A Multi-Centric Study
|
Madhuri Thakar, 1
Vandana Saxena,1
Nalini Janakiram,2
V Ravi,3 Anita
Desai,3 Surjit
Singh,4 Niranjan
Shivanna,2
Ranjana Minz,4
Arun Singh,5
Mitali Chatterjee,5
Manisha Madkaikar,6
Shilpa Bembalkar,1
Ajit Mukherjee,7 Vasantha
Mahesh7
From 1ICMR-National AIDS Research Institute, Pune,
Maharashtra; 2Indira Gandhi Institute of Child Health, and
3National Institute of Mental Health and Neurosciences,
Bengaluru, Karnataka; 4Post Graduate Institute of Medical
Education and Research, Chandigarh; 5Institute of Post
Graduate Medical Education and Research, Kolkata, West
Bengal; 6ICMR-National Institute of Immunohematology,
Mumbai, Maharashtra; and 7Indian Council of Medical
Research, New Delhi.
Correspondence to: Dr Madhuri Thakar, Head & Scientist-F,
Department of Immunology & Serology, ICMR, National AIDS
Research Institute, Plot No. 73, G-Block, MIDC, Bhosari,
Pune-411026, Maharashtra, India.
Email:
[email protected]
Received: August 20, 2020;
Initial review: September 22, 2020;
Accepted: January 13, 2021.
|
Objective: To determine the reference
ranges of various lymphocyte subsets in healthy Indian
children.
Design: Descriptive cross-sectional
study.
Setting: Four centers in India
representing four geographical regions.
Participants: 1104 children from
neonatal age to 18 years of age. Measurement: One
time measurement of absolute count and percentages of
different lymphocyte subsets i.e. T lymphocytes (CD3+T,
CD4+T, CD8+T cells), B lymphocytes (CD19+B cells) and
Natural Killer lymphocytes (CD15/16+NK cells) in whole blood
using multicolor flow cytometry.
Results: The absolute cell counts of
various lymphocytes were found to increase from newborn to
10 months of age, followed by gradual decline until 18
years; however, the proportion of immune cells remained
largely similar. Gender did not have a significant impact on
the reference ranges, whereas counts were found to vary as
per the geographical locations.
Conclusions: These reference ranges
will be useful to monitor and predict the immune status in
pediatric population. The variation in region wise ranges
could be confirmed by testing more number of samples in the
specific age groups.
Keywords: Flow cytometry, NK cell, CD4+T cells, B
cells.
|
C
ellular differentiation pathways in
children are distinctly different form adults [1].
Additionally, the cellular immune component of the blood is
known to be dynamic and showing variable frequencies of
different immune subsets at different ages especially in
pediatric population [7]. In India, although the lymphocytic
reference ranges are available for healthy adults [2], there
is not much data available on the reference range of
lymphocytic subsets among pediatric population [3]. Since
the ethnicity, age and environmental factors are known to
influence the lymphocytic reference ranges [4-6], the
available reference ranges from other countries cannot be
used for the Indian population. Considering the variations
in ethnicity across various geographic regions in India, it
is important to generate the reference values in different
pediatric age groups from across the country
In this study, we determined the age
group specific values for major lymphocyte subsets among
healthy pediatric population aged from newborn to 18 years
across different geographical regions in India.
METHODS
This cross-sectional study was aimed at
determining the reference values of lymphocyte subsets in
healthy Indian children aged 0 through 18 years from four
geographically diverse sites i.e., Bengaluru, Chandigarh,
Mumbai and Kolkata, in order to obtain the data
representative of the entire country. The different age
groups included in the study were: Group I- Newborn; Group
II - 6 weeks of age (before first DPT vaccination); Group
III - 9 to 10 months of age (before measles vaccination);
Group IV - 15 to 18 months of age or before first booster of
DPT; Group V - 19 months to 5 of age, Group VI - > 5 to 12
years of age; and, Group VII - 12 to 18 years of age. The
immunization visits coincided with the blood collection
visits.
For group I, cord blood was used as a
sample. For this group, babies with full term normal vaginal
delivery or elective caesarean with or without mild anemia
in pregnancy were included. Emergency caesarean cases,
complicated deliveries with chronic illness or with
infections, conditions like diabetes, toxemia, bleeding,
fever in mother, prolonged rupture of membrane, and HIV
positive pregnancy were excluded from this study.
For other groups (Group II to VII), the
inclusion criteria for healthy children in the different age
group were: no history of cold and cough (for the last one
month), blood transfusion (preceding 3 months), surgery
(preceding 6 months) and recent diarrhea (4-6 weeks), born
to HIV negative mother, and grade 1 malnutrition/ normal
weight (weight-for-age >70th centile IAP chart). Children
with moderate to severe anemia, acute or chronic infectious
diseases (gastrointestinal diseases within the last 6
months) or any clinically significant disease or findings in
the medical history that might compromise the study measures
(e.g., diabetes mellitus, asthma, rheumatoid arthritis,
cystitis fibrosis) were excluded from the study.
Children were enrolled after obtaining
written consent from their parents, and assent, if required.
The study was approved by the ethics committees of
respective study sites. From each site, 50 children were
enrolled in each group and in each age group an attempt was
made to enroll boys and girls in a 1:1 ratio. The enrollment
for groups I, II, III and IV was done in the hospitals
(well-baby clinics of the hospitals) and for groups V, VI
and VII, school-going healthy children were enrolled after
obtaining appropriate permission from the school. In one
day, not more than 5-7 eligible newborns/infants/ children
were enrolled in each group (Groups I to IV). For the groups
V, VI and VII, the schools were contacted and the eligible
participants were enrolled sequentially. The data on age,
sex, place of origin, height, weight, nutritional status and
vaccination was collected wherever possible.
Two to five milliliter of whole blood
specimens were collected from the children in K3 EDTA
evacuated tubes and were processed for immune-phenotyping
the same day. To avoid diurnal variation, the samples were
uniformly collected in the forenoon at all the study sites.
Immunophenotyping: The enumeration of
different lymphocyte subsets were done by multicolor flow
cytometry. The single platform technology was used to obtain
both the absolute counts and percentages. All the centers
used the same reagents, equipments and the standard
operating procedure to obtain comparable data. Briefly, in
the two separate Trucount tubes, 50
mL of
whole blood and 20 mL
of liquid antibody reagents (CD3 FITC, CD8 PE, CD45 PerCP,
CD4 APC) or (CD3 FITC, CD16+56 PE, CD45 PerCP, CD19 APC) was
added. All reagents were from the Becton Dickinson. The
tubes were incubated at room temperature in dark for 15
minutes. Lysis of the red blood cells was carried out using
450 ml
of 1:10 diluted FACS lysing solution). A total of 100000
cells were acquired in a FACSCalibur (BD Bio-sciences) and
analyzed using Multiset software (BD Biosciences). The
absolute count and percentage of the lymphocyte subsets in
the gate CD45high/SSClow
i.e., the count or the percentage from the total lymphocyte
population was calculated by the Multiset software. B
lymphocytes were identified as CD19+, T lymphocytes as CD3+
and further differentiated as CD4+ and CD8+ T cells and NK
cells were identified as CD3-CD16/CD56+cells
The optical alignment of the equipment
and fluorescence compensation settings were ensured daily by
running the calibration beads (CaliBRITE 3) and the
compensation was done using the FACSComp software.
Additionally, each center successfully participated in
National external proficiency testing programmed for CD4
count estimation.
Data analyses: To determine normal
ranges of lymphocyte parameters, 2.5 and 97.5 percentile
values were calculated, which covers 95% of the population
[8]. The age, gender and region specific ranges were also
reported. Any differences in lymphocyte subsets within the
geographic regions were assessed using Kruskal-Wallis Chi
square test. The region-wise value for each parameter in
each age group were compared with the overall reference
range of the respective parameter using Mann-Whitney U test.
P value of <0.05 was considered as significant.
Analyses were done using IBM SPSS 24.0.
RESULTS
A total of 1674 children were enrolled
across the four regions. Of these, data collected from 1104
subjects was considered for analysis; 570 samples were
excluded due to various reasons like quality of samples,
fail to fit in hemoglobin, BMI or weight criterion etc.
Region- and age-wise numbers of subjects in each group are
shown in Table I. The representation of the samples
in groups II (6.1%) and III (11.3%) were lower as compared
to the other groups. The median (range) weight
and hemoglobin of the newborns was 3 (2.5-4.5) kg and
16.1(13-22.1) g/dL, respectively. The hemoglobin decreased
to 11.8 (11-15.1) g/dL in group II, but remained similar in
older age groups. The median (range) body mass index was
16.6 (13.9-19.6) in group II, which remained similar in
older age groups.
Table I Regional Distribution of the Study Participants (N=1104)
|
East |
North |
South |
West |
|
n=317a |
n=281b |
n=304c |
n=202d |
| Newborn, n=194 |
50 (25.8) |
51 (26.3) |
49 (25.3) |
44 (22.7) |
| 6-32 wk, n=67 |
23 (34.3) |
39 (58.2) |
0 |
5 (7.5) |
| 9-10 mo, n=125 |
49 (39.2) |
24 (19.2) |
49 (39.2) |
3 (2.4) |
| 15-18 mo, n=132 |
46 (34.8) |
28 (21.2) |
47 (35.6) |
11 (8.3) |
| 19 mo-5 y, n=197 |
54 (27.4) |
44 (22.3) |
50 (25.4) |
49 (24.9) |
| 5-12 y, n=210 |
44 (21) |
55 (26.2) |
59 (28.1) |
52 (24.8) |
| 12-18 y, n=17a |
51 (28.5) |
40 (22.3) |
50 (27.9) |
38 (21.2) |
| No. of boys in
each region: a176, b138, c145 and d101. |
The median and 2.5th and 97.5th
percentiles of absolute counts and frequencies (%
populations) of various lymphocyte subsets; CD3+, CD4+ and
CD8+ T cells, B cells and NK cells in seven different age
groups are presented in Table II and Fig. 1.
Table II Median and Reference Range of Different Immune Cells in Indian Healthy
Children of Different Age Groups (N=1104)
|
Newborn |
6 -32 wk |
9 -10 mo |
15-18 mo |
19 mo-5 y |
5-12 y |
12-18 y |
|
n=194 |
n=67 |
n=125 |
n=132 |
n=197 |
n=210 |
n=179 |
| CD3+cells |
|
|
|
|
|
|
|
| Absolute counts |
2731 |
3421 |
4630 |
3801 |
3110 |
2347 |
1960 |
|
(979-5024) |
(952-8586) |
(1623-8159) |
(1480-6475) |
(1191- 6692) |
(1191-4497) |
(1035-4493) |
| Percentage |
65 (42-85) |
63 (38-78) |
63 (45-76) |
64 (37-77) |
63 (51-75) |
67 (51-77) |
66 (54-89) |
| CD4+cells |
|
|
|
|
|
|
|
| Absolute counts |
1827 |
2156 |
2852 |
2271 |
1821 |
1266 |
1080 |
|
(601-3243) |
(659-6132) |
(913-5680) |
(817-4893) |
(794-4323) |
(618-2555) |
(582-2045) |
| Percentage |
43(23-58) |
44 (15-60) |
39 (24-58) |
38 (24-53) |
39 (26-50) |
37 (26-50) |
36 (26-520) |
| CD8+cells |
|
|
|
|
|
|
|
| Absolute counts |
881 |
970 |
1407 |
1319 |
1084 |
913 |
767 |
|
(337-1889) |
(159-3717) |
(455-3393) |
(549-2844) |
(315-2258) |
(422-1878) |
(405-2615) |
| Percentage |
20 (11-40) |
18 (7-42) |
20 (10-37) |
22 (10-40) |
23 (13-32) |
26 (17-38) |
26 (17-52) |
| CD4/ CD8 ratio |
2.0 (0.8-4.6) |
2.4 (0.5-7.0) |
2.0 (0.8-5.5) |
1.7 (0.6-3.7) |
1.8 (0.9-3.3) |
1.4 (0.8-2.4) |
1.4 (0.6-2.4) |
| CD19+cells |
|
|
|
|
|
|
|
| Absolute counts |
760 |
1654 |
1915 |
1484 |
1187 |
653 |
507 |
|
(70-2532) |
(351-5946) |
(523-3799) |
(246-4139) |
(362-2754) |
(295-1650) |
(115-1117) |
| Percentage |
18 (4-43) |
27 (11-44) |
27 (13-42) |
26 (8-42) |
25 (16-37) |
19 (11-33) |
18 (4-29) |
| CD16+/56+ cells |
|
|
|
|
|
|
|
| Absolute counts |
499 |
489 |
433 |
368 |
335 |
362 |
334 |
|
(125) |
(114-1624) |
(105-1088) |
(114-1201) |
(131-1163) |
(124-1005) |
(78-774) |
| Percentage |
12 (4-36) |
8 (2-18) |
6 (2-16) |
6 (3-15) |
7 (3-17) |
10 (4-26) |
11 (3-24) |
|
All values in median (RR); RR-Reference ranges (2.5-
97.5 percentile). |
The absolute counts of CD3+, CD8+, CD4+ T
cells and CD19+B cells increased during the first few months
till 9-10 months and decreased gradually from 15-18 months
onwards till 12-18 years while NK cells showed a gradual
decline in the absolute count post 6 weeks of birth till
15-18 months of age and then plateaued (Fig. 1a). The
percentage values of CD3+, CD8+, CD4+ T cells (Fig. 1b)
along with the ratio of CD4 and CD8 largely remained
unchanged across different pediatric age groups. The
percentage of CD19+ B cells however increased from 6 weeks
to 5 years and later decrease in age group of 5-12 years and
further in 12-18 years of age. The percentage of NK cells
started to decline from 6 weeks onwards till 5 years of age
and later increased and reached to the levels present in new
born babies (Fig. 1b).
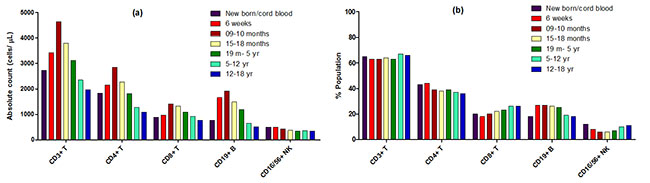 |
|
Fig. 1 The median values
of each lymphocyte subsets in study groups. (a)
Median values of absoplute counts, and (b)
proportion of CD3+ CD4+, CD8+, CD19+ and CD16-56+ NK
cells in all seven study groups.
|
The female: male ratio was similar across
different age groups (range: 1: 0.9 to 1: 1.19). The
age-specific overall ranges (Table II) were compared
with the gender-wise ranges in each age group for each
parameter. We found no significant difference in the
reference values observed in male and female children for
any parameter in any age group.
Similarly we also compared the
region-wise reference values with the overall reference
ranges within every age group for each parameter. The
significant difference was observed in case of group I
(newborn), group IV (15th 18 months of age), group V (19
months to 5 years), group VI (5 years to 12 years) and group
VII (12 years to 18 Years) for a few parameters. In newborn
group, the values were significantly different in all
lymphocyte subsets where as for other groups the values were
different for eg in CD3, CD8 and CD19 percentages and
absolute CD4 counts. The NK cell values were generally
similar showing difference only in group I in case of North
and South regions and in group VII in East and South regions
(Suppl. Table I). Due to insufficient number of study
participants from West region in groups II, III and IV, and
from South region in groups II, the comparisons could not be
made.
DISCUSSION
In this multi-centric study, we
determined the reference ranges for different lymphocyte
subsets in Indian pediatric population. This study
represents the largest dataset for the relative frequencies
of major lymphocyte subsets in healthy Indian children at
various age groups from birth till 18 years of age. Unlike
CD4+ and CD8+ T cells reference ranges, limited information
is available on other lymphocyte subsets like CD3+T cells,
CD19+ B cells and CD16/56+ NK cells which have important
immune functions.
Our observations confirmed the previous
findings that the lymphocyte compartments of normal healthy
children differ considerably in various age groups [8-11].
The absolute T cell and B cell count increased during the
first few months till 9-10 months and decreased gradually
from 15-18 months onwards till 12-18 years. Whereas the
relative percentages of T cells i.e. CD3+, CD8+ and CD4+
cells remained more or less similar in all age groups.
Similar findings have been reported in African and Caucasian
populations previously [10], and from children from southern
India [3].
We found that the reference ranges in our
cohort differ from pediatric population from other regions
like Europe [6,8,12], Africa [13,14], and North America
[11,15]. Among the newborns, the absolute cell counts for
CD3+, CD4+, CD8+, CD19+ B cells were lower than the cohort
from Italy [8] but higher than the African cohort from
Cameroon [14]. These differences could be due to the
differences in the total lymphocyte percentages and absolute
counts, which were not measured in both the studies.
Similarly the percentages of CD3+, CD4+ cells of the
newborns were lower than Italian children [8] but higher
than Cameroon [14], while the percentages of CD8+ T and
CD19+ B cells were higher in our newborn group than the
newborns from Italy and Cameroon. The number of samples
tested from the newborn group might be the reason for such
differences. The Italian study used 16 samples from the 0-3
month group whereas the Cameroon study used 38 cord blood
samples. In other age groups of children in our study, the
absolute CD4 counts were higher than seen in children from
Europe, Africa and USA; however in children from 6 years to
18 years, it was comparable to children from Uganda [16].
Except in newborns, children in all age groups had a higher
CD8 cell counts when compared with the children from Europe
(Italy), Africa (Tanzania, Uganda, Cameroon) and USA
[8,11,14,16]. The CD19+ B cell and NK cell counts were
higher than the counts observed from Italian population [8]
and largely comparable with the pediatric population from
Cameroon [14]. Rathore, et al. [17] compared the
different immune cells subsets in newborns from United
States and India and found that Indian newborns had higher
NK and CD4+ T cells, while lower subsets of total T cells,
than the American cohort. In comparison with these values,
the data from the present study showed lower CD4 counts
whereas the CD8+ T cells, B cells, NK cell counts were in
similar ranges. Similar to the absolute count, the
percentages of different immune cells also varied in our
pediatric cohorts in comparison to that of Europe, Africa
and USA [8,14,16,18]. These data collectively indicate that
each immune cell subset in different age groups of children
varies with the ethnicity and is influenced by the
geographical region. The lymphocyte subsets are known to
vary with the time of collection, use of different
equipments, procedure for estimation, and the time between
the collection and testing [8,9]. Hence, to minimize the
variation within the laboratories, proper quality control
measures were taken such as use of standard procedure,
sample collection in the forenoon hours at all the study
sites to avoid diurnal variation, and uniformity of
equipment and reagents across the sites. This pediatric
cohort did not show significant differences between the
sexes, as also observed in other studies [10]; although,
reference ranges for the CD4 count and percentages in Indian
adults were significantly higher in women [2].
India is a geographically heterogeneous
country, hence it was important to assess whether the
reference ranges differ in different geographical regions.
Our study showed significant differences between the
region-wise ranges (mostly East and South) in various
parameters in different age groups. These differences might
be due to the environmental, genetic or nutritional factors
[19-21]. Since this data could not be obtained, these
observations need to be confirmed on the larger sample size
from the specific age-groups. Moreover, the established
ranges could be reconfirmed on a small subset from time to
time as described earlier [18]. One of the limitations of
our study is insufficient samples available in some regions
for children belonging to groups II and III. This could be
the due to less number of babies coming for DPT immunization
during the study period. It would have been interesting to
examine the activation and functional profile of these
cells; however, due to the limitation of the parameters that
can be tested by the available flow cytometer, it could not
be evaluated but could be an important area of future
research.
In summary, this study provides reference
values for different lymphocyte subsets in Indian children
of varying age groups. Age was the only important variable
affecting the counts, and sex and geographical distribution
did not prove to be significant variables. This data can
find application in immune system evaluation of children of
Indian origin irrespective of sex, geographical distribution
and ethnicity. These age related reference ranges will be
helpful to assess the immune defects, and
suppression/absence of one or more immune functions in
Indian children with primary and secondary
immunodeficiencies as well as in autoimmune diseases.
Note: Supplementary material related
to this study is available with the online version at
www.indianpediatrics.net
Ethics clearance: ICMR-NARI Ethics
Committee; No. NARI/Age Lymphocyte subsets/10-11/100, dated
22 June, 2010.
Contributors: MT: Study
design, data analysis, preparation and review of manuscript;
VS: analyzed the data, drafted and reviewed the manuscript;
NJ,VR,AD,SS,NS,RM,AS,MC,MM: study design, execution of
study, patient information, data analysis, manuscript review
(at different sites); SS: data analysis, manuscript
preparation and review; AM: study design, data analysis
manuscript review; VM: study design, execution of the study
and review of the program. All authors approved the final
version of manuscript, and are accountable for all aspects
related to the study.
Funding: Indian Council of Medical
Research, India.
Competing interests: None
stated.
|
WHAT THIS STUDY ADDS?
•
Reference ranges are
provided for different lymphocyte subsets in
pediatric population from different geographical
locations in India.
|
REFERENCES
1. Tollerud DJ, Ildstad
ST, Brown LM, et al. T-cell subsets in healthy teenagers:
transition to the adult phenotype. Clin Immunol Immunopathol.
1990;56:88-96.
2. Thakar MR, Abraham PR, Arora S, et al.
Establishment of reference CD4+ T cell values for adult
Indian population. AIDS Res Ther. 2011;8:35.
3. Swaminathan S, Hanna LE, Raja A, et
al. Age-related changes in blood lymphocyte subsets of south
Indian children. Natl Med J India. 2003;16:249-52.
4. Webster HK, Pattanapanyasat K,
Phanupak P, et al. Lymphocyte immunophenotype reference
ranges in healthy Thai adults: implications for management
of HIV/AIDS in Thailand. Southeast Asian J Trop Med Public
Health. 1996;27:418-29.
5. Heldrup J, Kalm O, Prellner K. Blood T
and B lymphocyte subpopulations in healthy infants and
children. Acta Paediatr. 1992;81:125-32.
6. Bunders M, Cortina-Borja M, Newell ML,
European Collaborative S. Age-related standards for total
lymphocyte, CD4+ and CD8+ T cell counts in children born in
Europe. Pediatr Infect Dis J. 2005;24:595-600.
7. Carr EJ, Dooley J, Garcia-Perez JE, et
al. The cellular composition of the human immune system is
shaped by age and cohabitation. Nat Immunol. 2016;17:461-68.
8. Tosato F, Bucciol G, Pantano G, et al.
Lymphocytes subsets reference values in childhood. Cytometry
A. 2015;87:81-85.
9. Sack U GF TA. Age-related lymphocyte
subset changes in the peripheral blood of healthy children –
A meta-study. Transfus Med Hemother. 2007;24:176-81.
10. Schatorje EJ, Gemen EF, Driessen GJ,
et al. Paediatric reference values for the peripheral T cell
compartment. Scand J Immunol. 2012;75:436-44.
11. Chinen J, Rosenblatt HM, Smith EO, et
al. Long-term assessment of T-cell populations in DiGeorge
syndrome. J Allergy Clin Immunol. 2003;111:573-79.
12. Garcia-Prat M, Alvarez-Sierra D,
Aguilo-Cucurull A et al. Extended immunophenotyping
reference values in a healthy pediatric population.
Cytometry B Clin Cytom. 2019;96:223-33.
13. Buchanan AM, Muro FJ, Gratz J, et al.
Establishment of haematological and immunological reference
values for healthy Tanzanian children in Kilimanjaro Region.
Trop Med Int Health. 2010;15:1011-21.
14. Sagnia B, Ateba Ndongo F, Ndiang Moyo
Tetang S, et al. Reference values of lymphocyte subsets in
healthy, HIV-negative children in Cameroon. Clin Vaccine
Immunol. 2011;18: 790-95.
15. Reichert T, DeBruyere M, Deneys V, et
al. Lymphocyte subset reference ranges in adult Caucasians.
Clin Immunol Immunopathol. 1991;60:190-208.
16. Lugada ES, Mermin J, Kaharuza F, et
al. Population-based hematologic and immunologic reference
values for a healthy Ugandan population. Clin Diagn Lab
Immunol 2004;11:29-34.
17. Rathore DK, Holmes TH, Nadeau KC, et
al. Differences in multiple immune parameters between Indian
and U.S. infants. PLoS One. 2018;13:e0207297.
18. Valiathan R, Deeb K, Diamante M, et
al. Reference ranges of lymphocyte subsets in healthy adults
and adolescents with special mention of T cell maturation
subsets in adults of South Florida. Immunobiology.
2014;219:487-96.
19. Semba RD, Muhilal, Ward BJ, et al.
Abnormal T-cell subset proportions in vitamin-A-deficient
children. Lancet. 1993;341:5-8.
20. Fawzi WW, Msamanga GI, Spiegelman D,
et al. Randomised trial of effects of vitamin supplements on
pregnancy outcomes and T cell counts in HIV-1-infected women
in Tanzania. Lancet. 1998;351:1477-82.
21. Choong ML, Ton SH, Cheong SK.
Influence of race, age and sex on the lymphocyte subsets in
peripheral blood of healthy Malaysian adults. Ann Clin
Biochem. 1995;32:532-39.
|
|
|
 |
|

