Renal biopsy is an
important diagnostic tool in the hands of a pediatric nephrologist.
While the first biopsy was done more than 100 years ago in United
States, its utility in diagnostics has increased in the last few decades
[1]. Since its regular introduction in 1951 by Iverson and Brun, renal
biopsy has made a revolution in the study of renal diseases [2].
Renal pathology can be better delineated with the advent of newer
stains, immunofluorescence and electron microscopy. While a renal biopsy
is more useful in diagnosing glomerular diseases, it often provides
information on tubular conditions as well.
Pre- procedure Care
The parents/ caregivers should be counseled and explained the
procedural details and a written consent should be taken. The
prerequisites for a biopsy are hemoglobin above 8 gm/dL,
platelet count above 1 lakhs/mm3,
normal INR and normal blood pressure. In the pre-biopsy
checklist, it is important to take history of bleeding
tendencies, allergies to povidone/iodine, ketamine, midazolam
and lidocaine. Drugs like aspirin should be discontinued seven
days before, warfarin 48 hr before and any other NSAIDs should
also be stopped 48 hours prior to the procedure. The biopsy site
should be inspected for any superficial infection. If the child
is on hemodialysis, the procedure should be done after at least
24 hours of last dialysis session as heparin during the dialysis
procedure may lead to excessive bleeding. For patients with
prolonged BT (>8-10 minutes, e.g., in SLE, azotemia), 0.3
µg/kg IV desmopressin can be administered 30 min prior, or 2-4
µg/kg DDAVP intranasal 2 hours before the procedure.
Desmopressin reduces the bleeding by improving the platelet
functions.
PROCEDURE
The renal biopsy is done under sedation and local anesthesia
in prone position for native kidneys and in supine position for
transplanted kidneys. Preferably the procedure should be done
under real-time ultrasound guidance by a pediatric
nephrologist/trainee in pediatric nephrology.
In conditions like abdominal distension and ascites the biopsy
can be done in lateral decubitus or sitting position. A
sandbag/rolled sheet or blanket is used under the abdomen to
decrease the mobility of the kidney. An IV access is established
and heart rate, saturation and blood pressure are monitored
during the procedure. For procedural sedation the most preferred
drugs are 1-2 doses of midazolam (0.1mg/kg) and ketamine 0.5-1.0
mg/kg. Intravenous atropine 0.01 mg/kg is administered 1-2
minutes after midazolam. The left renal angle is the most
preferred site for the renal biopsy. The lower pole of the
kidney is located at this position. Local anesthesia is given at
the site by infiltration of lignocaine injection after draping
and cleaning (with spirit and povidone iodine). In the real time
procedure under ultrasound guidance the automated biopsy gun
should be introduced at the site in such a manner that its tip
reaches the renal cortex.
Technique
The sample is
usually obtained from the lower pole of the left kidney located
between the erector spinae muscle and the lower border of the
12th rib. A 16 or 18 gauge biopsy gun/automated needle is used
for taking the per-cutaneous renal sample. Use of an 18 gauge
needle is preferred in infants and young children while thicker
bore should be used in all other age groups. The yield of
glomeruli is better with 16 gauge needle [3]. The use of an 18
gauge needle resulted in a significantly smaller sample size (9
vs 11 and 15 glomeruli) and less diagnostic success (53%
vs 76% and 85%), with no significant differences in
complication rates [3]. The sample should be taken from the
renal cortex which harbors the glomeruli. The cortical thickness
in an adult kidney is about 10 mm.
A renal sample is considered
adequate for opinion if the yield of glomeruli is between 10-20.
Minimum sample size for diagnosis varies greatly with the
specific diagnosis. For instance, membranous glomerulonephritis
(MGN) can be diagnosed even from a single glomerulus while focal
segmental glomerulosclerosis (FSGS) can be missed if less than
10 glomeruli are obtained. Two to three passes with the
automated gun are sufficient to yield tissue for light
microscopy, immunofluoresence (IF) study and electron microscopy
(EM). The sample should be removed from the biopsy needle with
gentleness, taking care not to stretch or crush the tissue.
Forceps should be avoided. An 18-gauge needle or a thin, wooden
stick, such as a toothpick can be used. It is advisable to
confirm adequacy of cortical tissue on the table itself with the
help of a pathologist using the stereoscopic microscope. Once it
is determined that suitable cortical tissue is obtained, about 2
mm tissue are cut off from each cortical and medullary ends of
the two cores (Fig. 1). One cortical tissue
and one medullary tissue is placed for IF study in a vial
containing Michel transport media. Antigens of interest in the
renal biopsy are protected for as long as a week in this media
and the sample is stable at room temperature. The cortical and
medullary samples for EM are sent in 1-3% glutaraldehyde that
acts as a fixative. This fixative must be refrigerated and has a
short half life. Care must be taken that no cross contamination
of fixative fluids occur while placing the biopsy pieces in
their respective vials.
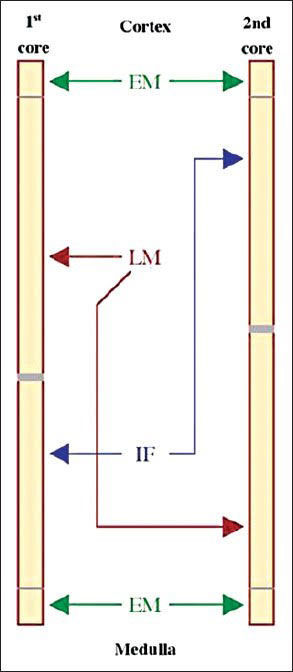 |
| Fig. 1
Division of kidney biopsy cores for light
microscopy, immunofluorescence and electron microscopy.
|
Complications
Complications of renal biopsy are few with the
use of automated gun. Macroscopic hematuria following a biopsy
has been reported to vary from 5-20% in different studies [4-6].
Rarely patients may develop colicky pain due to passage of clots
in urine. Although clinically significant
peri-nephric hematomas occur in less than 6% of the biopsies,
peri-nephric hematomas have been demonstrated at 24-72
hours after biopsy in >90% of cases evaluated prospectively.
Microscopic hematuria occurs in almost all patients and
disappears over a 48-72 hours period. Serious complications like
need for blood transfusion and development of an arteriovenous
fistula are less frequent. A meta-analysis on complications
following renal biopsy in children reported the need for blood
transfusion in 0.9% and need for another intervention due to the
procedure in 0.7% of the biopsied children [7]. Absolute
contraindications of the procedure are uncontrolled bleeding
diathesis, uncontrolled severe hypertension,
hydronephrotic kidneys while presence of a single kidney
is a relative one.
Post Biopsy Care
Patient should stay in supine position for 4-6 hours and bed
rest is recommended for 24 hours. The vitals should be monitored
every 30 min for the first 2 hours and then hourly till 6 hours.
Maintenance intravenous fluids (normal saline or N/2 saline or
ringer lactate) are administered for the first 6 hours. Oral
fluids are offered to the child when fully conscious and on
demand. Paracetamol is used for pain relief if required. Most
patients can be discharged after 24 hours of biopsy; however
they should be instructed to avoid climbing of stairs, heavy
work and play for one week following the procedure.
Treatment of Complications:
(i) Gross hematuria:
If coagulation is deranged it is recommended to use fresh
frozen plasma or cryoprecipitate for reduction of bleeding. Also
an extra dose of vitamin K should be administered. Blood
transfusion may be necessary if 6 hour post biopsy hemoglobin
falls by 10-15% of the baseline or the child clinically becomes
pale. An urgent ultrasound abdomen should be done to visualize
bleed, hematoma in such a situation. Rarely radiographic
transcatheter embolization or surgical intervention may be
required for continuous bleeding. (ii) Sedation related
complications: Brief hypoxia, transient airway compli-cations,
vomiting or minor aspiration, laryngospasm are complications of
sedation and might need repositioning of the child with
suctioning of the airways, oxygen administration and rarely
ventilation.
INDICATIONS
The primary indication for renal biopsy in a child is steroid
resistant nephrotic syndrome (SRNS) [8-9]. A recent
retrospective review showed that 36.1% of the pediatric biopsies
were for SRNS, 22.1% for steroid sensitive disease and 12% for
acute kidney injury (AKI) [10]. Glomerular diseases (62.6%)
predominated in the national Turkish registry review of all
pediatric biopsies between 1991 and 2010 [11].
The most likely biopsy findings in patients with
nephrotic syndrome are minimal change disease (MCD), FSGS and
mesangioproliferative glomerulonephritis (Mes PGN) [12]. While
MCD predominates in younger children, FSGS is more common in
older children and adolescents [13]. Biopsy findings of
membranoproliferative glomerulonephritis (MPGN) and MGN occur in
less than 5% of patients with steroid resistant disease in
children. Primary glomerular diseases accounted for almost 85%
of all biopsies in older children in a recent study [13]. The
indications for renal biopsy are listed in Box I.
|
Box I Common Indications for Renal Biopsy in
Children |
|
Glomerular causes
Steroid
resistant nephrotic syndrome
Congenital nephrotic
syndrome
Atypical nephrotic syndrome
Rapidly
progressive glomerulonephritis
Non resolving
post-infectious glomerulonephritis.
Recurrent gross
hematuria
HBSAg/anti HCV positivity with
proteinuria/hematuria
Tubulo-interstitial
nephritis
Acute kidney injury >4 wks without
cause
|
All children with steroid sensitive or resistant nephrotic
syndrome require a biopsy prior to starting calcineurin
inhibitors (cyclosporine and tacrolimus) which are potentially
nephrotoxic [9]. Besides children with nephrotic syndrome on
calcineurin inhibitors for more then 2-3 years are often
re-biopsied to look for features of nephrotoxicity before
further continuation of these agents.
Other indications of biopsy are in patients with rapidly
progressive renal failure where a suspicion of crescentic
glomerulonephritis is kept. In patients with acute nephritic
syndrome, renal biopsy is needed if the kidney functions are
worsening or the investigations are not suggestive of a post
streptococcal glomerulone-phritis.
Renal biopsy may be done in patients with AKI to identify
the underlying cause where recovery is delayed beyond one month
to differentiate acute tubular necrosis from other causes of
AKI. Other conditions like acute and chronic interstitial
nephritis can be identified on kidney biopsy. While kidney
biopsy is not required for the diagnosis of chronic kidney
disease (CKD) it may be done where the kidneys appear normal in
size and corticomedullary differentiation on sonography and the
cause of CKD is not explicable. In post transplant patients, the
biopsy of the grafted kidney provides information on acute and
chronic rejection. Biopsy of kidneys with structural anomalies
should be done carefully under ultrasound guidance. In this
article we would be discussing the biopsy interpretation of some
common glomerular conditions occurring in native kidneys.
INTERPRETATION
Light Microscopy
For light microscopic examination of renal biopsy specimen,
stains used include hematoxylin-eosin stain (HE stain), periodic
acid Schiff (PAS) stain, Masson trichrome and silver stains.
Identification of cortex or medulla, number of glomeruli, and
cells infiltrating the interstitium of the kidney like
neutrophils and lymphocytes are best identified on the HE stain.
For glomerular structure, PAS stain is better as it delineates
mesangial cells and matrix. PAS and silver stains effectively
stain the basement membrane while Massons trichrome and silver
stains are used for identification of fibrosis.
The number of glomeruli, size, presence of any sclerosis, focal
or diffuse changes, and presence of any crescents or mesangial
cell proliferation can be checked on light microscopy. Lesions
involving <50% of glomeruli are called focal while more than
that are called diffuse. If only a part of the glomerulus is
involved it is termed segmental while involvement of the whole
glomerulus is defined as global. Basement membrane thickening or
splitting is seen in conditions like MGN and MPGN and is
identified on PAS or silver stain. Vessel wall thickening,
medial sclerosis or fibrinoid necrosis in case of vasculitis is
better seen with PAS stain [14-16]. Stains like von Kossa for
calcification and Congo red for identification of amyloidosis
are used infrequently in specialized situations [14]. Chronic
tubulointerstitial damage can be identified on light microscopy
as tubular atrophy and interstitial fibrosis. In acute
interstitial nephritis, interstitial edema, infiltration by
neutrophils, lymphocytes and plasma cells can be seen while in
chronic interstitial nephritis; fibrosis instead of edema is a
prominent feature [2].
Immunofluorescence
IF study is done with labeled antisera and antibodies.
Antisera or monoclonal antibodies against immunoglobulins (IgA,
IgG and IgM), components of the classical or alternative
complement pathway (C1q, C3c and C4d), protein light chains
(kappa and lambda), albumin and fibrinogen are used for
identification of different immunofluoresence patterns. The
pattern of staining can be linear or granular; linear staining
occurs in anti-GBM disease while granular in immune complex
mediated injury. The location of deposits can be mesangial or in
the peripheral capillary walls (PCW).
In conditions like MPGN and MGN, the immuno-glogulin (IgG)
deposits are primarily subendothelial and subepithelial
respectively. Mesangial deposits of IgA are primarily seen in
IgA nephropathy. Similarly granular C3 deposits in the PCW are
consistent with a diagnosis of post infective glomerulonephritis
(PIGN) while deposits of all immunoglobulins and complements
(full house staining) are a hallmark of lupus nephritis.
Using immunohistochemistry procedures, antibodies against
viruses like cytomegalovirus and polyoma virus can identify
these in the biopsy specimens. Antibodies against hepatitis B
and C antigens can be detected on renal tissue and nature of
amyloid whether primary or secondary identified by AA Amyloid
stain. Additional immunohistochemical study with antibodies,
such as collagen IV alpha chains can be performed for
identification of Alports syndrome. In post transplant renal
biopsies immunostaining for complement factor C4d can be done to
identify humoral rejection.
Electron Microscopy
It is not necessary, but helpful to do EM for all renal
biopsies. The conditions in which electron microscopy will help
confirm the light microscopy diagnosis are in the identification
of podocyte structure alteration (effacement of foot processes)
in MCD, changes of glomerular basement membrane especially
thinning, thickening or splicing and the site of immune deposits
(subendothelial or subepithelial). EM is essential for diagnosis
of basement membrane abnormalities like thinning in thin
basement membrane disease and irregular thickening with basket
weave pattern in Alports syndrome.
EM is also essential in sub defining the nature of
deposits in immune complex deposition diseases like
immunotactoid glomerulonephritis (GN) and fibrillary GN.
Metabolic disease like Fabry disease also require EM for
diagnosis. Box II gives in a nutshell what to look
for in a renal biopsy specimen.
|
Box II Features to Look for in Renal Biopsy with
Different types of Processing |
Light microscopy
Glomerular proliferation or sclerotic changes can be
identified best
Basement membrane thickening can be
identified
Tubulointerstial damage like tubular
atrophy and fibrosis can be seen
Blood vessels may
show medial sclerosis
Immunoflorescence
Helps in identifying immune deposits like C3, IgG, IgM,
IgA, fibrin etc.
Electron microscopy
Most useful in identifying the structural defects of
podocytes like effacement in MCD, identifying the exact
location of immune deposits (subepithelial or
subendothelial), basement membrane thickening or
thinning (in Alport disease and thin basement membrane
disease) |
BIOPSY PICTURE IN GLOMERULAR DISORDERS
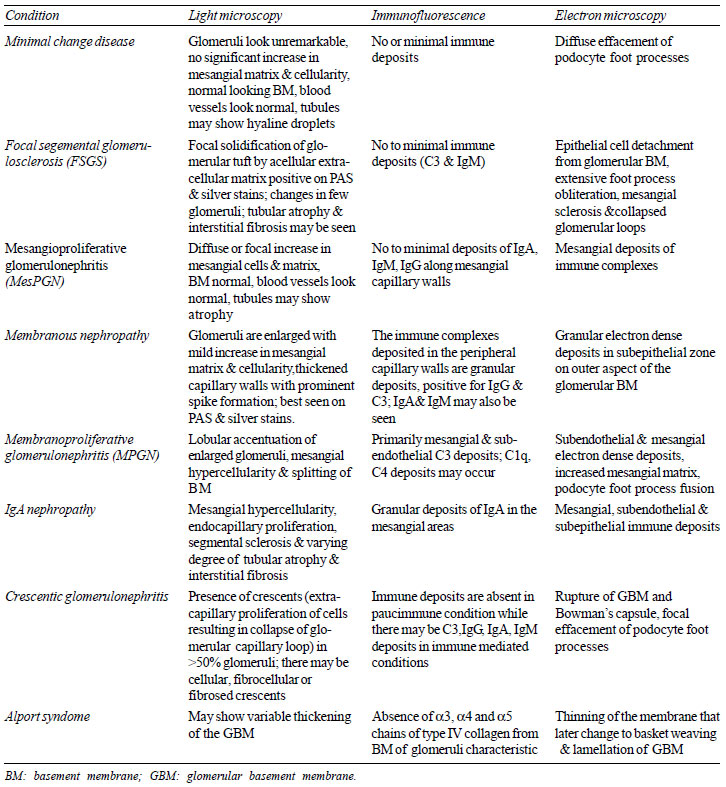
Some salient biopsy characteristics of renal disorders are
given in Table I; the biopsy picture in different
conditions is discussed briefly below.
Table I Salient Features on
Renal Biopsy in Different Conditions
|
 |
MCD:
The glomeruli in MCD look
almost unremarkable. There is no significant increase in
mesangial matrix and cellularity and no thickening of basement
membrane is identified. Tubules may show hyaline droplets
represen-tative of resorbed proteins following the heavy
protei-nuria. Immunofluorescence studies are generally nega-tive
for all immunoglobulins (Fig. 2 a, b). MCD
is part of set of diseases called as podocytopathies
characterized by abnormalities in the podocytes or visceral
epithelial cells lining the glomerular capillary loops. There is
simplification of foot processes of podocytes seen as diffuse
effacement on electron microscopy (Fig. 2c);
which is the hallmark of the disease.
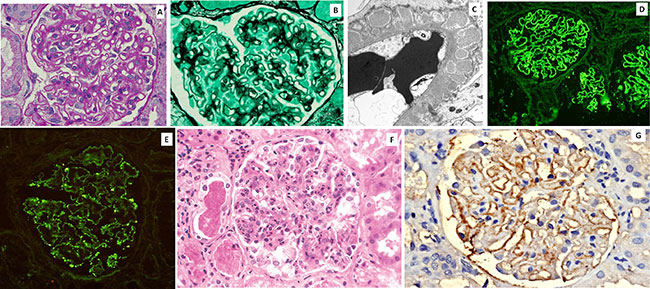 |
| Fig. 2 Minimal
Change Disease: A:PAS stained section of a glomerulus in
Minimal Change Disease showing only mild increase in
mesangial matrix (x400x); B: IgG stained cryosection
showing lack of immune deposits and protein reabsorption
granules in parietal epithelial cells (x200x); C:
Electron micrograph showing complete effacement of foot
processes of podocytes (x4300x). Focal and Segmental
Glomerulosclerosis: D: HE stained glomerulus in focal
and segmental glomerulosclerosis showing segmental
sclerosis of glomerular tuft and foam cell infiltration
[arrow] (x400x); E: JSM Stained section showing
segmental sclerosis in glomerulus (x400x); F: Electron
micrograph showing complete effacement of foot processes
of podocytes (x4300x); G: C3 stained cryosection showing
segmental deposits in glomerular tuft (x200x). |
FSGS:
The classical lesion in FSGS is a focal solidification of the
glomerular tuft by an acellular extracellular matrix that is
positive on PAS and silver stains (Fig. 2d,e).
The segmental sclerosis is often accompanied by attachment to
the Bowmans capsule called as synechie formation. These
lesions are identified in only a portion of the glomeruli and do
not involve the entire glomerular tuft; hence the term focal and
segmental. FSGS can further be pathologically classified as
glomerular tip, perihilar, cellular, collapsing and mixed
variants according to Columbia classification. On IF study,
segmental glomerular staining for IgM and C3 is identified which
represents a non specific entrapment in the area of sclerosis.
Staining for immunoglobulins is generally negative (Fig.
2f). EM shows effacement and obliteration of podocyte foot
processes, mesangial sclerosis (Fig. 2 g).
MGN:
This is a disease caused by
immune complex deposition in the sub-epithelial zone i.e. over
the basement membranes of the capillary loops. The capillary
basement membranes show spike formation due to deposition of
type IV collagen around this material in an attempt to wall them
off and decrease their inflammatory reaction
(Web Fig. 1a, b).
The glomeruli are enlarged with mild increase in mesangial
matrix and cellularity. Activity in the form of endocapillary
proliferation or crescent formation are not a feature of primary
MGN but usually representative of a membranous nephropathy
secondary to a systemic cause like SLE, other auto immune or
infectious diseases. On IF,
the immune complexes deposited in the peripheral
capillary walls are classically identified as granular deposits,
positive for IgG and C3 (Web Fig.
1d). Other immunoglobulins like IgA and IgM
are often seen. Further a diagnosis of primary MGN may be
confirmed by demonstration of anti-PLA2R
antibodies in the podocytes by immuno-fluorescence staining (Web
Fig. 1e). On EM, granular electron dense
deposits are identified in the sub-epithelial zone on the outer
aspect of the glomerular basement membrane (Web
Fig. 1d).
MPGN:
This term indicates thickening
of the basement membrane accompanied by mesangial proliferation.
The kidney biopsy in MPGN shows a classical lobular accentuation
of glomeruli, mesangial hypercellularity and splitting of
basement membranes on silver stains. Secondary MPGN pattern of
injury is seen in cases of long standing infectious pathology,
auto-immune conditions, dysproteinemias, transplant
glomerulopathy and various other miscellaneous conditions.
Primary MPGN is caused by abnormalities of the alternate
complement pathway and is known as C3 glomerulo-pathy. It is
primarily diagnosed on IF by strong deposition of C3 in the
kidney biopsy in the absence of immunoglobulin deposition. The
diagnosis can only be confirmed on EM, based on which, it is
further divided into dense deposit disease (DDD) and C3
glomerulonephritis (C3GN). Dense, band-like osmophilic deposits
in the GBM on EM is the classical feature of DDD. C3GN is
characterized by sub-endothelial, mesangial and sub-epithelial
C3 deposits on EM.
IgA nephropathy:
IgA nephropathy is
one of the commonest forms of primary glomerulonephritis the
world over. It is characterized by granular deposits of IgA in
the mesangial areas identified on IF. On light microscopy, these
biopsies present a diverse histological presentation ranging
from no detectable histological finding to diffuse proliferative
and crescentic glomerulonephritis. The grade of histological
changes determines the clinical prognosis. The histological
changes in the form of mesangial hypercellularity, endocapillary
proliferation, segmental sclerosis, tubular atrophy and
interstitial fibrosis have been graded by the Oxford
classification into 4 grades each (0-4). A sum total of grades
in all the four compartments represents the activity of the
disease and determines the clinical prognosis [17].
Lupus nephritis:
The renal biopsy in lupus nephritis shows a wide variety of
changes which commensurate with the disease activity and have a
bearing on the prognosis of the patient. The renal biopsy
changes in lupus have been classified into 6 groups by the ISN
/RPS classification system into Class I (minimal lupus
nephritis), Class II (mesangial lupus nephritis), Class III
(focal lupus nephritis), Class IV (diffuse lupus nephritis),
Class V (membranous lupus nephritis) and Class VI (advanced
sclerosing glomerulonephritis). Some modifications have been
added to the classification [18].The diagnosis is confirmed on
IF by presence of a full house pattern in the form of
immunoglobulins IgG, IgA and IgM along with complements C3 and
C1q; deposits of immunoglobulins are also indentified in the
walls of tubules and blood vessels.
Tubulointerstitial
changes:
The tubules should be examined for features of acute tubular
necrosis as seen in AKI. The interstitium shows edema and a
mixed inflammatory cell infiltrate. Other findings are
interstitial fibrosis, tubular atrophy, arteriolar sclerosis,
and occasionally, patchy mononuclear cell infiltration. The
degree of chronic parenchymal damage in the tubulo-interstitial
compartment is an important prognostic indicator in all
glomerular diseases and is assessed on PAS and MT stains. Blood
vessels changes secondary to hypertension are often seen in
glomerular diseases.
To conclude, interpretation of renal
biopsy in children involves procuring an adequate sample for
examination and processing it for light, immunofluorescence and
electron microscopy. While renal biopsy is more useful for
identifying glomerular diseases, it also provides sufficient
information about tubulo-interstitial changes. The biopsy
changes should be carefully interpreted along with the clinical
findings for making a confirmatory diagnosis.
Funding:
None; Competing interests: None stated.
REFERENCES
1. Korbet
SM. Nephrology and the percutaneous renal biopsy: A
procedure in jeopardy of being lost along the way. Clin J Am Soc
Nephrol. 2012;7:1545-7.
2. Luciano RL, Moeckel GW.
Update of Native kidney biopsy: Core Curriculum 2019. Am J
Kidney Dis. 2019;73:404-15.
3. Nicholson ML,
Wheatley TJ, Doughman TM, White SA,
Morgan JDT, Veitch PS, et al. A prospective
randomized trial of three different sizes of core-cutting needle
for renal transplant biopsy. Kidney Int. 2000;58:390-5.
4. Roy RR, Mamun A,
Shamshul Haque SM, Rahman M. Role of renal biopsy in manging
pediatric renal diseases. A midterm analysis of a series at
Bangabandu Sheikh Mujib University, Dhaka, Bangladesh. Saudi J
Kidney Dis Transpl. 2017;28:125-32.
5. Sadaf A1, Khemchand
MN, Fouzia L, Asia Z. Clinicopathological profile of
pediatric renal biopsies at a tertiary care hospital, Pakistan.
Saudi J Kidney Dis Transpl. 2018;29:1403-9.
6. Rianthavorn P, Kerr
SJ, Chiengthong K. Safety of paediatric percutaneous native
kidney biopsy and factors predicting bleeding complications.
Nephrology (Carlton). 2014;19:143-8.
7. Varnell CD Jr, Stone
HK, Welge JA. Bleeding complications after pediatric
kidney biopsy: A systematic review and meta-analysis. Clin J Am
Soc Nephrol. 2019;14: 57-65.
8. Bagga A., Indian
Pediatric Nephrology Group, Indian Academy of Pediatrics.
Revised guidelines for management of steroid-sensitive nephrotic
syndrome. Indian J Nephrol. 2008;18:31-9.
9. Indian Society of
Pediatric Nephrology, Gulati A, Bagga A, Gulati S, Mehta
KP, Vijayakumar M. Management of steroid resistant nephrotic
syndrome. Indian Pediatr. 2009;46: 35-47.
10. Rico MP, Cuellar CR,
Hernandez MF, Chaparro LSG, Agredo OLP, Gastelbondo RA.
Characterization and etiopathogenic approach of pediatric
renal biopsy patients in a Colombian medical center from
2007-2017. Int J Nephrol. 2018; 28:2018.
11. Fidan K, Isik Gonul
I, Büyükkaragöz B, Isiyel E, Arinsoy T, Soylemezoglu O. Changing
trends in pediatric renal biopsies: analysis of
pediatric renal biopsies in national nephrology registry data.
Ren Fail. 2016; 38:1228-33.
12. Churg J, Habib R, White
RH. Pathology of the nephrotic syndrome in children. A report
for the International Study of Kidney Disease in Children.
Lancet 1970; 760:1299-302.
13. Muthu V, Ramachandran
R, Nada R, Kumar V, Rathi M, Kohli HS, et al.
Clinicopathological spectrum of glomerular diseases in
adolescents: A single-center experience over 4 Years. Indian J
Nephrol. 2018;28:15-20.
14. Fogo AB. Core
curriculum in nephrology: Approach to renal biopsy. Am J Kidney
Dis 2003;42:826-36.
15. Amann K, Haas CS. What
you should know about the work up of a renal biopsy. Nephrol
Dial Transplant.
2006;21:1157-61.
16. Regele H, Mougenot B,
Brown P, Rastaldi MP, Leontsini M, Gesualdo L, et al.
Report from Pathology consensus meeting on renal biopsy handling
and processing, Vienna, February 25, 2000. Available at:
http://www.kidney-euract.org/Rbpathologyconsensus. htm.
Accessed February 9, 2020.
17. Cattran DC, Coppo R,
Cook HT, Feehally J, Roberts ISD, Troyanov S, et al. The
Oxford classification of IgA nephropathy: Rationale,
clinicopathological correlations and classification. Kidney Int.
2009;76:534-45.
18. Markowitz GS, DAgati VD. The ISN/RPS 2003 classification of
lupus nephritis: An assessment at 3 years. Kidney Int.
2007;71:491-5.

