|
|
|
Indian Pediatr 2018;55:404-404 |
 |
Early
Neurodevelopmental Outcomes After Corrective Cardiac Surgery In
Infants
|
|
Ritchie Sharon Solomon 1,
Tanuja Sasi2,
Abish Sudhakar1,
Raman Krishna Kumar1
and
Balu Vaidyanathan1
From Departments of 1Pediatric Cardiology
and 2Pediatrics, Amrita Institute of Medical Sciences, Amrita
University, Kochi, Kerala, India.
Correspondence: Dr Balu Vaidyanathan, Clinical
Professor, Pediatric Cardiology, Amrita Institute of Medical Sciences,
Amrita University, Kochi, Kerala 682 041, India.
baluvaidyanathan@gmail.com
Received: March 10, 2017;
Initial review: June 19, 2017;
Accepted: February 07, 2018.
|
Objective: To assess neurodevelopmental status in
Indian infants undergoing corrective surgery for congenital heart
disease (CHD) and to analyze factors associated with neurodevelopmental
delay.
Design : Cross-sectional study.
Setting: Tertiary-care pediatric cardiology
facility.
Participants: Consecutive infants undergoing
corrective surgery for CHD (January 2013 –December 2014). Palliative
procedures, and patients with known genetic syndromes were excluded.
Main outcome measures: Neurodevelopmental
evaluation 3 months, and one year after surgery using Developmental
Assessment Scales for Indian Infants (DASII); scores were categorized as
delayed if £70.
Results: Of the 162 children enrolled, delayed
PDI and MDI scores were observed in 33.5% and 19.6% of patients at 3
months, respectively; this reduced to 14.5 % on 1-year follow-up. On
multivariate analysis, delayed PDI outcome at one year was predicted by
early term birth and one-year postoperative head circumference Z-score
<–2. Delayed MDI was associated with higher mean perfusion pressure on
cardiopulmonary bypass. Cardiac diagnosis and peri-operative factors did
not impact neurodevelopmental outcomes.
Conclusions: Neurodevelopmental status is delayed
in 14.5% of infants one year after corrective infant heart surgery.
Keywords: Congenital heart disease, Neuromotor delay, Outcome
|
|
O
utcomes of surgery for congenital heart disease
(CHD) have been improving in recent times [1]. Studies from developed
countries have reported higher prevalence of neurological and speech
impairments as well as deficits in attention and executive functions on
follow-up [2-6].
There is limited information about neurodevelop-mental
outcomes after surgery for CHD from low- and middle-income countries
(LMICs). Of late, many centres from LMICs have started performing
corrective surgical procedures in neonates and infants with CHD, with
outcomes comparable to those from developed nations [7,8]. However, the
demographic profile and pre-operative characteristics are very different
in LMICs and these could significantly impact long-term outcomes [7-12].
An analysis of Indian children with uncorrected CHD has shown that they
are at increased risk of developmental delay [13]. These can have a
significant impact on neurodevelopmental outcomes despite high- quality
surgical expertise and post-operative intensive care.
This prospective study from a tertiary-care pediatric
cardiac center examines the short term neuro-developmental outcomes in
infants undergoing corrective surgery for CHD.
Methods
This cross-sectional study was conducted in a
tertiary-care pediatric cardiology facility in Kerala, from January 2013
to December 2014. Consecutive patients (<1 year) undergoing corrective
surgery for CHD were included. Exclusion criteria included: (i)
palliative operations, (ii) Genetic syndromes, (iii)
preterm babies (<37 weeks gestation), (iv) small for gestational
age (birth weight <10 th
percentile/<–2SD), and (v) confirmed neuro-logical or
developmental abnormalities. The study protocol was approved by the
Institutional ethics committee and written informed consent was obtained
from all parents.
Demographic details included age at surgery, sex,
birth weight, gestational age (early term 37- 39 weeks vs. full term
³39 weeks),
mode of delivery, and socioeconomic class as per modified Kuppusamy
classification [14]. Anthropometric data (weight, height, head
circumference and weight/height) were taken pre-operatively and at
follow-up. Z-scores were calculated based on World Health Organisation
(WHO) normograms for age and sex with values <–2 considered as abnormal.
Cardiac diagnosis with the pre-operative risk score
based on the Risk Adjustment for Congenital Heart Surgery 1 (RACHS-1)
classification was recorded. Details of prenatal diagnosis, delivery and
mode of transport to the cardiac center (monitored/unmonitored) were
recorded. Duration of ventilation and prostaglandin (PGE1) infusion
(hours), ICU and hospital stay (days), hematocrit, sepsis (culture
positive/clinical), oxygen saturation and nature of the surgical
procedure (planned/urgent) were recorded.
Duration of cardiopulmonary bypass (CPB), aortic
cross clamp (ACC) and total circulatory arrest (minutes), mean perfusion
pressure during CPB (mm Hg), minimum hematocrit, lowest temperature,
modified/continuous ultrafiltration, major hypoxic events (PaO2 <55 mm
Hg), cardiac arrest, arrhythmia with hemodynamic compromise, air
embolism and need for re-institution of CPB were noted. The CPB flow
rates and mode of ultra-filtration were based on surgical preferences.
Direct cerebral vascular monitoring was not done.
Duration of mechanical ventilation and inotropic
support (hours), ICU and hospital stay (days) were noted. Delayed
sternal closure, re-intubations, pulmonary hypertension (PH) crisis,
cardiac arrests requiring resuscitation, focal neurological deficits,
post-operative sepsis and seizures were noted. Prolonged ventilation,
and prolonged ICU stay were defined as >48 hours and >7 days,
respectively.
All patients were followed up 3 months and 1 year
after surgery. Neurodevelopmental assessment was done using
Developmental Assessment Scale for Indian Infants (DASII), which is
considered to be the best formal test in Indian context [15]. Using
DASII, the psychomotor developmental index (PDI) and mental
developmental index (MDI) were calculated and categorized as delayed
when scores were £70
(£–2 SD).
Early intervention program was initiated if the PDI and MDI were delayed
after the first follow-up assessment.
Statistical analysis: Student’s t
test was used to compare continuous risk factors by normal and delayed
group. Chi-square test was used to find the association between
demographic and perioperative categorical factors by PDI and MDI (delay)
category. One way ANOVA was used to compare the PDI and MDI by RACHS
category. Multivariate binary logistic regression analysis (stepwise)
was used to estimate the Odds Ratio with 95% Confidence Intervel and
adjusting for potential confounding variables. The cut-off point for
statistical significance was set an alpha level of 5%. Statistical
analysis was done using IBM SPSS 20.0 (SPSS Inc, Chicago, USA).
Results
Of the 162 infants (92 males) included, 52 (32.1%)
were neonates. There was no in-hospital mortality. Four patients (2.4%)
died on follow-up; 3 had residual cardiac issues, and in one cause of
death was unknown (Fig. 1). RACHS category 1 and 2
constituted 59.3% (96) of the surgical procedures (Web
Table I). Prenatal diagnosis was made in 4.3% of patients.
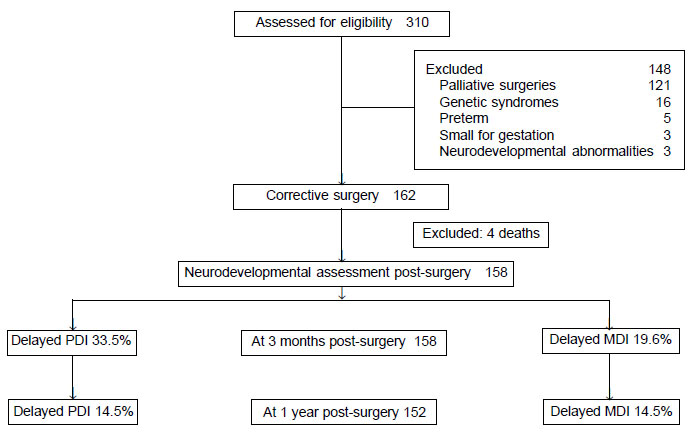 |
|
Fig. 1 Flow of participants in the
study.
|
The distribution of acyanotic (80, 49.4%) and
cyanotic CHD were similar. Preoperative weight, height, head
circumference were abnormal in 110 (67.9%), 48 (29.6%) and 60 (37%)
children, respectively. Preoperative ICU care was needed in 56 (36.4%)
with a median stay of 4 (1-30) days; 15 (9.3%) had pre-operative sepsis.
The median age at surgery was 60 days (2-365).
Major intraoperative complications occurred in 10
infants (6.2%). One had a major hypoxic event while 9 patients (5.5%)
had arrhythmias with hemodynamic compromise. Re-institution of CPB was
required in 7 infants (4.3%). The median CPB and ACC time were 109 min
(41-457) and 58 min (10-274), respectively (Web Table
I). The mean (SD) hematocrit and perfusion pressure on CPB was 28.4
(3.11) %and 38.4 (4.11) mm Hg. The median duration of postoperative
ventilation and inotrope was 42 (3 - 552) and 72 (2 - 432) hours,
respectively. Delayed sternal closure was done in 8 infants (4.9%). Mean
(SD) postoperative ICU stay was 7.3 (5.41) days. Postoperative sepsis
occurred in 32 infants (19.8%). Re-intubation was required in 15 (9.3%)
infants.
Anthropometric indices improved on one-year follow-up
with abnormal weight for age and weight/height Z scores in 23% (35) and
16.4% (25), respectively. Height-for-age and head circumference Z scores
were abnormal in 27.6% (42) and 23% (35), respectively.
At three months after corrective surgery, delayed PDI
was seen in 53 (33.5%) and mean (SD) PDI score was 81.2 (33.02); this
improved to 92.4 (26.02) at one-year follow-up (22, 14.5% delayed). On
univariate analysis, factors associated with delayed PDI at one-year
follow-up included gestational age <39 weeks, weight and head
circumference Z-score <–2 on follow-up, postoperative ventilation
>48 hours, postoperative ICU stay >7 days and longer duration of
postoperative inotrope use. Lower mean PDI score was associated with
weight (P=0.02) and head circumference Z-score < -2 (P=0.009)
at one year follow up.
At three months after corrective surgery, delayed MDI
was seen in 31 (19.6%) and mean (SD) MDI score was 90.0 (27.17); this
improved to 96.1 (26.05) at one-year follow-up (22, 14.5% delayed). On
univariate analysis, factors associated with lower mean MDI scores at
one-year follow-up were gestational age <39 weeks (P<0.001) and
preoperative weight Z-score <-2 (P=0.03).
On multivariate regression analysis, the
variables associated with delayed PDI outcome at one year follow-up were
gestational age <39 weeks and one year postoperative head circumference
Z score <-2 (Table I). Delayed MDI at one year was
associated with higher mean perfusion pressure on CPB (P = 0.05).
TABLE I Multivariate Regression Analysis of Predictors of Delayed PDI and MDI
|
Variables |
OR (95% CI) |
P value |
|
PDI outcome at 1 year follow-up |
|
Gestational age < 39 wk |
7.51 (1.61-35.03) |
0.01 |
|
#Head circumference <–2 Z score |
5.39 (1.19-24.47) |
0.02 |
|
MDI outcome at 1 year follow up |
|
Mean perfusion pressure on CPB |
1.13 (1.00-1.27) |
0.05 |
|
CPB: Cardiopulmonary bypass; #at 1 year post-surgery. |
Discussion
We analyzed the short-term neurodevelopmental
outcomes in Indian infants undergoing corrective surgery for CHD. Though
the PDI and MDI scores improved on one-year follow-up, 14.5% of patients
continued to have delay. The motor skills was more affected than the
mental skills and cognitive function at both points of time.
Patient-specific constitutional factors like early term birth and head
circumference on follow-up were associated with neurodevelopmental
outcomes rather than peri-operative factors or cardiac diagnosis.
Formal developmental assessment by DASII was not done
pre-operatively and it is possible that subtle neurological
abnormalities which could have impacted outcomes, and pre-existing
developmental delay were missed. We did not have a cohort of non-CHD or
unoperated infants for comparison of developmental indices.
The patterns of developmental delay are in accordance
with findings of previous studies affecting psychomotor domains more
than mental milestones [3,6,13,17]. Several studies have stated that
patient-specific factors are important determinants of
neurodevelopmental outcome compared to peri-operative factors [2,3,18].
Recent studies have reported the impact of gestational age at delivery
on outcomes of neonatal heart surgery [19]. Our data suggests better
neurodevelopmental outcomes for infants born at full term compared to
early term deliveries [5]. There is a common practice to plan a early
(<39 weeks) delivery once a prenatal diagnosis of critical CHD is made
to facilitate expedited postnatal cardiac care [20]. Delivery before
full term may have a greater impact on brain development and
neurodevelopmental outcomes [21,22]. Postnatal studies in term neonates
with complex CHD have shown smaller head circumferences compared with
normal term neonates [21-23]. Possible reasons attributed to this
include cerebral hypo-oxygentation, shared genetic or environmental or
placental factors [23]. It is possible that some of these factors may
persist despite surgical correction of the CHD, thus influencing
neurodevelopmental outcomes. Studies have shown that head circumference
at birth is a predictor of head circumference at 1 year of age, thereby
explaining our observation of lower head circumference at 1-year
follow-up predicting lower PDI [3,24]. Cerebral perfusion pressure, mean
cerebral blood flow velocity and regional cerebral oxygen saturation
index (rSO2i) would have been better indicators of the adequacy of
cerebral blood flow on CPB and in predicting hypoxic ischemic and
reperfusion injury [25].
Our results suggest that a pre-operative
neurodevelopmental evaluation needs to be done for all patients
undergoing surgery for CHD. Neuro-developmental evaluation should be
included in the follow-up of all patients, and early intervention
program should be initiated whenever deficits are detected.
Neurodevelopmental clinics need to function in collaboration with a
multidisciplinary team comprising of developmental pediatrician,
neurologist, pediatric cardiologist and occupational therapist [26].
Longer follow-up is needed to assess the overall development of these
children in higher intellectual domains and executive functions.
In conclusion, psychomotor developmental and mental
developmental scores are delayed in 14.5% of infants one year after
corrective infant heart surgery and are dynamic in nature.
Patient-specific factors like early term birth and lower head
circumference at one-year after surgery predicted neurodevelopmental
outcomes than cardiac factors.
Contributors: RSS: designed the study,
collected and analyzed data and drafted the initial manuscript. TS: did
the neurodevelopmental follow up; AS: did the data analysis and revised
the manuscript; RKK: contributed to the study design and revised the
manuscript; BV: conceptualized and designed the study, analyzed the
data, reviewed and edited the manuscript and shall act as the guarantor
for the manuscript.
Funding: None; Competing interests:
None stated.
|
What is Already Known ?
• Children with congenital heart disease are
at risk for neuro-developmental delay even after corrective
surgery.
What This Study Adds?
• Patient-specific factors like early term
birth and head circumference at one-year after surgery predict
neurodevelopmental outcomes than peri-operative factors or the
cardiac diagnosis in children undergoing cardiac surgery for
congenital heart disease.
|
References
1. Simpson JM. Impact of fetal echocardiography. Ann
Pediatr Cardiol. 2009;2:41-50.
2. Forbess JM, Visconti KJ, Bellinger DC, Howe RJ,
Jonas RA. Neurodevelopmental outcomes after biventricular repair of
congenital heart defects. J Thorac Cardiovasc Surg. 2002;123:631-9.
3. Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J,
Gerdes M, Zackai E, et al. Patient characteristics are important
determinants of neurodevelopmental outcome at one-year of age after
neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg.
2007;133:1344-53.
4. Forbess JM, Visconti KJ, Hancock-Friesen C, Howe
RC, Bellinger DC, Jonas RA. Neurodevelopmental outcome after congenital
heart surgery: Results from an institutional registry. Circulation.
2002;106 (12 Suppl 1) 95-102.
5. Goff DA, Luan X, Gerdes M, Bernbaum J, D’Agostino
JA, Rychik J, et al. Younger gestational age is associated with
worse neurodevelopmental outcomes after cardiac surgery in infancy. J
Thorac Cardiovasc Surg. 2012;143:535-42.
6. Fuller S, Nord AS, Gerdes M, Wernovsky G, Jarvik
GP, Bernbaum J, et al. Predictors of impaired neuro-developmental
outcomes at one year of age after infant cardiac surgery. Eur J
Cardiothorac Surg. 2009;36:40-8.
7. Bakshi KD, Vaidyanathan B, Sundaram KR, Roth SJ,
Shivaprakasha K, Rao SG et al. Determinants of early outcome
after neonatal cardiac surgery in a developing country. J Thorac
Cardiovasc Surg. 2007;134:765-71.
8. Reddy NS, Kappanayil M, Balachandran R, Jenkins
KJ, Sudhakar A, Sunil GS, et al. Pre-operative determinants of
outcomes of infant heart surgery in a limited resource setting. Semin
Thorac Cardiovasc Surg. 2015;27:331-8.
9. Vaidyanathan B, Radhakrishnan R, Sarala DA,
Sundaram KR, Kumar RK. What determines nutritional recovery in
malnourished children undergoing correction of congenital heart defects?
Pediatrics. 2009;124:e294-e9.
10. Vaidyanathan B, Roth SJ, Rao SG, Gauvreau K,
Shivaprakasha K, Kumar RK. Outcome of ventricular septal defect repair
in a developing country. J Pediatr. 2002;140:736-41.
11. Kumar RK, Shrivastava S. Pediatric heart care in
India, Heart. 2008;94:984-90.
12. Changlani TD, Jose A, Sudhakar A, Rojal R,
Kunjikutty R, Vaidyanathan B. Outcomes of infants with prenatally
diagnosed congenital heart disease delivered in a pediatric cardiac
facility. Indian Pediatr. 2015;52:852-6.
13. Lata K, Mishra D, Mehta V, Juneja M. Neuro-developmental
status of children aged 6-30 months with congenital heart disease.
Indian Pediatr. 2015;52:957-60.
14. Ravi Kumar BP, Dudala SR, Rao AR. Kuppuswamy’s
socio-economic status scale – a revision of economic parameter for 2012.
Int J Res Dev Health. 2013; 1:2-4.
15. Patni B. Developmental assessment scales for
indian infants. Ind J Pract Pediatr. 2012;14:409-12.
16. Visootsak J, Mahle WT, Kirshbom PM, Huddleston L,
Caron-Besch M, Ransom A, et al. Neurodevelopmental outcomes in
children with Down syndrome and congenital heart defects. Am J Med Genet
A. 2011;155:2688-91.
17. Gaynor JW, Stopp C, Wypij D, Andropoulos DB,
Atallah J, Atz AM, et al. Neurodevelopmental outcomes after
cardiac surgery in infancy. Pediatrics. 2015;135:816-25.
18. Newburger JW, Sleeper LA, Bellinger DC, Goldberg
CS, Tabbutt S, Lu M, et al. Early developmental outcome in
children with hypoplastic left heart syndrome and related anomalies: the
Single Ventricle Reconstruction Trial. Circulation. 2012;125:2081-91.
19. Costello JM, Pasquali SK, Jacobs JP, He X, Hill
KD, Cooper DS, et al. Gestational age at birth and outcomes after
neonatal cardiac surgery: an analysis of the Society of Thoracic
Surgeons Congenital Heart Surgery database. Circulation.
2014;129:2511-7.
20. Bartlett JM, Wypij D, Bellinger DC, Rappaport LA,
Heffner LJ, Jonas RA, et al. Effect of prenatal diagnosis on
outcomes in D-transposition of the great arteries. Pediatrics.
2004;113:e335-e40.
21. Miller SP, McQuillen PS, Hamricketal S. Abnormal
brain development in newborns with congenital heart disease. N Engl J
Med. 2007;357:1928-38.
22. Andropoulos DB, Hunter JV, Nelson DP, Stayer SA,
Stark AR, McKenzie ED, et al. Brain immaturity is associated with
brain injury before and after neonatal cardiac surgery with high-flow
bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg.
2010;139:543-56.
23. Matthiesen NB, Henriksen TB, Gaynor JW, Agergaard
P, Bach CC, Hjortdal VE, et al. Congenital heart defects and
indices of fetal cerebral growth in a nationwide cohort of 924 422
liveborn infants. Circulation. 2016;133:566-75.
24. Gunn JK, Beca J, Hunt RW, Goldsworthy M,
Brizard CP, Finucane K, et al. Perioperative risk factors for
impaired neurodevelopment after cardiac surgery in early infancy. Arch
Dis Child. 2016;101:1010-16.
25. Brady KM, Mytar JO, Lee JK, Cameron DE, Vricella
LA, Thompson WR, et al. Monitoring cerebral blood flow pressure
autoregulation in pediatric patients during cardiac surgery. Stroke.
2010;41:1957-62.
26. Marino BS, Lipkin PH, Newberger JW, Peacock G,
Gedes M, Gaynor JW, et al. American Heart Association Congeni-tal
Heart Defects Committee, Council on Cardiovascular Disease in the Young,
Council on Cardiovascular Nursing and Stroke Council. Neurodevelopmental
Outcomes in Children with Congenital Heart Disease: Evaluation and
Management. A Scientific Statement from the American Heart Association.
Circulation. 2012;126:1143-71.
|
|
|
 |
|

