|
|
|
Indian Pediatr 2018;55: 395-399 |
 |
Anti-HBs Titers Following Pentavalent
Immunization (DTwP-HBV-Hib) in Term Normal Weight vs Low
Birthweight Infants
|
|
Chandrika Verma 1,
MMA Faridi1,
Manish Narang1
and Iqbal R Kaur2
From Departments of 1Pediatrics and 2Microbiology,
University College of Medical Sciences and Guru Teg Bahadur Hospital,
Delhi, India.
Correspondence to: Dr MMA Faridi, B-14, G-4, Vivek Vihar Phase-1,
Delhi 110 095.
Email: mmafaridi@yahoo.co.in
Received: December 18, 2016;
Initial review: March 06, 2017;
Accepted: January 15, 2018.
Published online:
February 09, 2018.
PII:S097475591600108
|
|
Objective: To compare anti-HBs
titers between term low birth weight (1800-2499 g) infants and normal
birthweight infants, 6 weeks after last dose of primary immunization
with pentavalent vaccine, and to study adverse events following
immunization (AEFI) with pentavalent vaccine.
Design: Cohort study.
Setting: Tertiary-care hospital
predominantly catering to urban poor population of East Delhi.
Participants: 265 low birthweight
(1800-2499 g) and 265 normal birthweight (2500-4000 g) infants.
Monovalent Hepatitis B vaccine was administered within 24 hours of birth
followed by three primary doses of pentavalent vaccine at 6, 10 and 14
weeks. Anti-HBs titers were estimated after 6 weeks of third dose of
pentavalent vaccine. Adverse events following immunization (AEFI) month
were observed for a month after each dose of pentavalent vaccine.
Main outcome measures: Anti HBs
antibody titers after 6 weeks of primary immunization, and AEFI.
Result: 443 (83.5%) infants (225
low birthweight and 218 normal birthweight infants) completed the
follow-up. Seroprotection against hepatitis B virus was achieved in both
groups after pentavalent vaccine administration. Anti HBs GMTs in low
birthweight infants (194.8 mIU/mL) and normal birthweight infants (204.2
mIU/mL) were comparable (P = 0.17). No serious adverse events
were observed in either group.
Conclusion: Three primary doses
of pentavalent vaccine administered along with zero dose of Hepatitis B
vaccine at birth provide good seroprotection. The vaccine appears to be
safe in both low birth weight and normal birthweight infants born at
term.
Keywords: Combination vaccines, Hepatitis B
vaccine, Immunogenicity, Low birthweight infants.
|
|
U
niversal immunization against hepatitis B in
infancy starting at birth has resulted in marked reduction in HBV
related chronic hepatitis, liver cirrhosis, and hepatocellular
carcinoma. An anti-HBs concentration of
³10 mIU/mL measured
1–3 months after administration of the last dose of the primary
vaccination is considered a reliable marker of protection against HBV
infection [1]. However, the antibody response to hepatitis B vaccine has
been shown to depend on the schedule of vaccination, birth weight,
gestation, chronological age, gender, genetic factors, co-morbidities
and the immune status of the vaccinee [2]. Preterm infants weighing
<2000g at birth may not mount an adequate response to hepatitis B
vaccine. The World Health Organization (WHO) recommends that the birth
dose of hepatitis B vaccine to such preterm infants should not be
counted and an additional dose of hepatitis B vaccine should be given to
them [3]. Term low birth weight (LBW) infants weighing 1800 to 2499 g;
with several of them being small for gestational age, may lie in the
grey zone of the immunity where they may be vulnerable despite being
born chronologically mature [4]. Immunogenicity of monovalent hepatitis
B vaccine in term low birth weight babies has been found to be
satisfactory [5,6]. However, there is inadequate data on the immunogenic
response of hepatitis B vaccine after immunization with pentavalent
vaccine (DTwP-HBV-Hib) among term LBW infants when ‘zero’ dose of
monovalent hepatitis B vaccine is also administered at birth. This study
compared anti HBs titres after 6 weeks of primary immunization with
pentavalent vaccine between term infants weighing 1800-2499 g at birth
and normal birth weight infants. Infants in both groups were also
observed for adverse events following pentavalent vaccine
administration.
Methods
This study was conducted in the Departments of
Pediatrics and Microbiology at University College of Medical Sciences
and Guru Tegh Bahadur Hospital, Delhi over a period of 17 months
(December 2013-April 2015) after approval from the Ethical Committee of
the institute and written informed consent from the parents.
Clinically healthy eligible neonates born
consecutively at term gestation were allocated in LBW (1800 to 2499 g)
and normal birthweight (2500 to 4000g) groups within 24 hours of birth
till desired sample size was reached. Infants born to hepatitis B
positive mothers, neonates suffering from sepsis, birth asphyxia,
meconium aspiration syndrome, gross congenital anomalies, requiring
exchange transfusion and whose families were planning to leave the area
before the period of completion of study were excluded.
Sample size was calculated based on the study by
Sharma, et al. [7] Wherein indigenous pentavalent vaccine
produced anti HBs geometric mean titers (GMT) of 616.7 mIU/mL in healthy
term infants. Expecting a difference of 15% in anti-HBs titers between
LBW and normal birthweight babies, at 80% power and 5% level of
significance, 476 infants were required, equally divided between LBW and
normal birthweight groups. Considering an estimated attrition rate of
10%, we planned to recruit 265 infants in each group (total 530
infants).
Pentavalent vaccine consisting of Diphtheria,
Tetanus, Pertussis, Hepatitis B, and Haemophilus influenzae type B
Conjugate vaccine adsorbed (Serum Institute of India Ltd, Pune) was
used. Each dose of 0.5 mL contained Diphtheria Toxoid 25 Lf (30 IU),
Tetanus Toxoid 2.5 Lf (40 IU), B. pertussis (whole cell) 16 OU (
4.0 IU), HBsAg (rDNA) 10 mcg and Purified capsular HiB Polysaccharide
(PRP) conjugated to Tetanus Toxoid (carrier protein) 10 mcg.
We collected 2 mL of cord blood in plain sterile vial
from placental end and stored at –20ºC after separation of serum.
Breastfeeding was initiated within 1 hr after normal delivery; and
within 2 hrs in babies delivered through caesarean section. Monovalent
recombinant Hepatitis B vaccine (dose 0.5 mL, 10 mcg purified HBsAg),
manufactured by Biological E Ltd, India, was administered in the
anterolateral aspect of thigh within 24 hours of birth, by a trained
staff nurse. BCG and OPV zero dose were also administered at the same
time. Birth weight, length and head circumference were recorded at birth
by standard methods.
Infants were called at 6 weeks (+2 weeks), 10 weeks
(+2 weeks) and 14 weeks (+2 weeks), and 0.5 mL pentavalent vaccine (DTwP-HBV-Hib)
was administered by intramuscular injection into the anterolateral
aspect of thigh by trained staff nurses. Trivalent OPV was also
administered simultaneously. All infants were monitored for 1 hour
following immunization for development of any adverse event.
Mother/guardian was given a proforma to record the adverse events at
home, and was advised to contact telephonically or return back on
occurrence of any serious adverse event. The proforma for adverse events
was checked at each follow-up visit and minor adverse events such as
fever and local tenderness were managed symptomatically. Weight, length
and head circumference were recorded at each visit by standard methods.
Mothers were counselled to bring the infants 6 weeks after the third
dose of pentavalent vaccine and 2 mL venous sample was collected in
plain vial; serum was stored at –20ºC.
Serum samples were thawed and anti-HBs titers were
estimated using enzyme linked immunosorbent assay (ELISA) based kits
(DIA.PRO, Diagnostic Bioprobes Srl, Italy). The calibrators and samples
were tested as per the protocol provided with the kit. Validation check
was carried out on the controls.
Statistical analysis: The GMTs were calculated by
taking the antilog of the mean of the logarithmic transformation of the
titers. Antibody titers between the two groups were compared using
unpaired student t-test. Proportion of infants developing adverse events
following immunization (AEFI) was assessed using chi-square test. The
analysis was carried out using SPSS 20.0 software.
Results
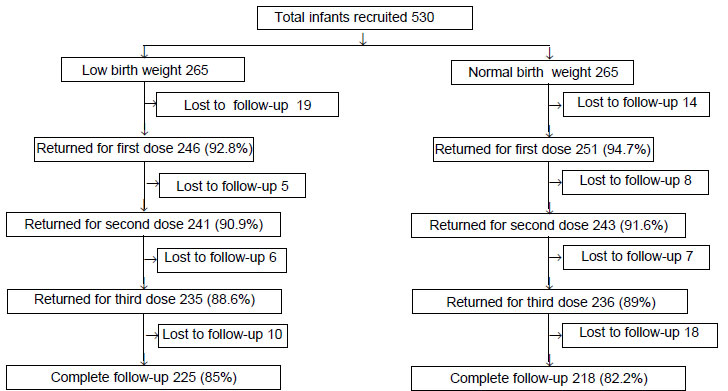
A total of 93.1% (443) infants completed follow-up
(LBW 94.5%; normal birth weight 91.6%) (Fig. 1).
Table I depicts the baseline characteristics of participants
in both groups.
 |
|
Fig. 1 Distribution of participants in
the study.
|
TABLE I Baseline Parameters of Enrolled Infants at Birth
|
Parameters |
Low birth
|
Normal birth
|
|
weight group
|
weight group
|
|
(n=225) |
(n = 218) |
|
Gestational age (wk)*
|
37.8 (0.7) |
39.4 (1.4) |
|
Birthweight (g)* |
2119 (187.9) |
2781.6 (269) |
|
Length (cm)* |
47.5 (0.9) |
49.7 (1.4) |
|
Head circumference (cm)* |
32.5 (0.8) |
33.9 (0.6) |
|
Cord blood Anti HBs titers#$ |
0.0 (0.0-0.0) |
0.0(0.0-0.0) |
|
*Values expressed as mean (SD) #Values expressed
as median (IQR); $in mIU/mL. |
The median (IQR) cord blood anti HBs levels of 443
infants was 0 (0,0). Minimum level of anti HBs titers observed after 6
weeks of primary immunization with pentavalent vaccine was 40 mIU/mL in
both the groups. Maximum anti-HBs titers attained were 280 mIU/mL and
282 mIU/mL, respectively in LBW and normal birth weight groups. Mean
(SD) anti-HBs titers were 206.76 (60) mIU/mL and 214 (55.46) mIU/mL,
respectively for LBW and normal birthweight infants (P=0.17).
Anti-HBs GMTs were 194.76 mIU/mL and 204.2 mIU/mL in LBW and normal
birthweight infants, respectively and the difference was not significant
(P=0.17).
Table II shows the adverse events observed in
443 infants of the two groups who completed three doses of pentavalent
vaccine. Common adverse events were fever, tenderness and induration.
All the adverse events resolved with symptomatic management. These
adverse events decreased with subsequent doses of immunization.
TABLE II Incidence of Adverse Events in Infants Receiving Pentavalent Vaccine (N=443)
|
Adverse event |
After 1st dose
|
P value |
After 2nd dose
|
P value |
After 3rd dose
|
P value |
|
LBW
|
NBW
|
|
LBW
|
NBW
|
|
LBW |
NBW |
|
|
(n=225) |
(n=218) |
|
(n=225) |
(n=218) |
|
(n=225) |
(n=218) |
|
|
Tenderness |
73 (32.4) |
62(28.4) |
0.4 |
55 (24.4) |
49 (22.5) |
0.6 |
37 (16.4) |
34 (15.6) |
0.8 |
|
Redness# |
|
|
|
|
|
|
|
|
|
|
Any |
54 (24) |
44 (20.2) |
0.5 |
24 (10.7) |
28 (12.8) |
0.2 |
12 (5.3) |
19 (8.7) |
0.1 |
|
Mild (<5 mm) |
10 (4.4) |
7 (3.2) |
|
8 (3.6) |
4 (1.8) |
|
0 (0) |
0 (0) |
|
|
Moderate (5-20 mm) |
40 (17.8) |
31 (14.2) |
|
16 (7.1) |
24 (11) |
|
12 (5.3) |
19 (8.7) |
|
|
Severe (>20mm) |
4 (1.8) |
6 (2.8) |
|
0 (0) |
0 (0) |
|
0 |
0 |
|
|
Induration# |
|
|
|
|
|
|
|
|
|
|
Any |
72 (32) |
73 (33.5) |
0.6 |
42 (19.1) |
43 (19.7) |
0.9 |
19 (8.4) |
20 (9.1) |
0.4 |
|
Mild (<5 mm) |
12 (5.4) |
10 (4.6) |
|
0 (0) |
0 (0) |
|
0 |
0 |
|
|
Moderate (5-20 mm) |
57 (25.3) |
56 (25.7) |
|
43 (19.1) |
43 (19.7) |
|
19 (8.4) |
20 (9.1) |
|
|
Severe (>20 mm) |
3 (1.3) |
7 (3.2) |
|
0 (0) |
0 (0) |
|
0 |
0 |
|
|
Fever |
|
|
|
|
|
|
|
|
|
|
Any |
103 (45.8) |
105 (48.1) |
0.6 |
105 (46.7) |
98 (44.9) |
0.3 |
50 (22.2) |
48 (22) |
0.7 |
|
100.1-101ºF |
43 (19.1) |
49 (22.5) |
|
50 (22.2) |
41 (18.8) |
|
31 (13.8) |
26 (12) |
|
|
101.1-102ºF |
40 (17.8) |
42 (19.2) |
|
50 (22.2) |
46 (21.1) |
|
19 (8.4) |
22 (10) |
|
|
>102ºF |
20 (8.9) |
14 (6.4) |
|
5 (2.2) |
11 (5) |
|
0 (0) |
0 (0) |
|
|
Vomiting |
16 (7) |
13 (5.6) |
0.7 |
9 (4) |
10 (4.2) |
0.8 |
5 (2.2) |
5 (2.3) |
1.0 |
|
Diarrhea |
12 (5.3) |
9 (4.13) |
0.6 |
5 (2.2) |
7 (3.2) |
0.5 |
0 (0) |
0 (0) |
|
|
Values expressed as n (%); No child had persistent cry, seizures
or anaphylaxis, excessive sleepiness or restlessness; #Assessed
at 1 hr. after vaccination. |
Discussion
We evaluated the immunogenicity of the hepatitis B
vaccine component of pentavalent vaccine in term low birthweight and
normal birthweight babies. In this study we found that all infants
irrespective of their birthweight, attained seroprotective titres
(anti-HBs ³10
U/mL). Baseline anti-HBs titers observed in the cord blood samples were
negligible, and after four doses of hepatitis B vaccine, given as
monovalent Hepatitis B vaccine at birth followed by three primary doses
of pentavalent vaccine, all infants achieved seroprotective levels of
anti HBs titers and Anti HBs GMTs were comparable in LBW and normal
birth weight infants delivered at term gestation.
The assumed difference of 15% in the anti HBs titres
between LBW and normal birth weight infants was arbitrary and this could
have affected the actual sample size. The other limitation of the study
was that the number of infants studied might not address safety and
tolerability of the vaccine. A good (93%) follow-up of the infants at 6
weeks after immunization is the strength of the study.
Earlier studies have shown concerns that LBW infants
have low levels of T and B lymphocytes and lower vaccine specific IgG
responses as compared to normal birth weight babies [8-10]. Studies
evaluating the immunogenicity of hepatitis B component of pentavalent
vaccine in term infants enrolled at 6 weeks of age have concluded the
vaccine to be immunogenic with seroprotection rates ranging from 97% to
100% [11-14]. Studies evaluating different brands of pentavalent (DTwP-HBV-
Hib) vaccine reported comparable anti HBs GMT in all infants [7,14,15].
Our study reiterates that pentavalent vaccine is highly immunogenic in
infants immunized with monovalent hepatitis B vaccine at birth.
Although, all infants in our study achieved seroprotective titers, the
anti HBs GMTs observed in our trial were lower than those reported in
the above studies in both normal birth weight and LBW infants. Different
pharmacological preparations and physical properties of the vaccine;
characteristics of ELISA testing kits and ethnicity may be the possible
reasons for different immunogenicity and levels of GMTs. However, this
difference does not seem to be clinically significant because anti HBs
seroprotective titre (>10 mIU/mL) is attained in all children.
There are studies documenting the good immunogenicity
of monovalent hepatitis B vaccine in both low birth weight and normal
birth weight infants [5, 6, 16, 17]. These results correlate well with
our results where term LBW infants attained good immune response and
reiterate the fact that pentavalent vaccine is as immunogenic as the
separately administered monovalent vaccine. A retrospective cohort study
in Nigeria analyzing the immunization records from June 2011 to May 2013
revealed the significant improvement in uptake of vaccines and
completion of the schedule when pentavalent vaccine was used as compared
to separately administered DPT and Hepatitis B vaccine [18]. The vaccine
was also safe and tolerable in these studies.
We conclude that three primary doses of pentavalent
vaccine administered along with zero dose of Hepatitis B vaccine at
birth achieved comparable seroprotective anti HBs GMT in LBW and normal
birth weight infants and that the immunization with pentavalent vaccine
appears to be safe.
Contributors: MMAF: Conceptualized the
study, supervised the work, and critically reviewed the manuscript and
will stand guarantor; CV: collected the data, searched literature,
carried out estimation of anti-HBs titers, drafted and analyzed the
results; MN: helped in search of the literature and reviewed the
manuscript; IK: critically analyzed the results and reviewed the
manuscript. All authors approved the final draft.
Funding: Directorate of Family Welfare,
Govt. of NCT of Delhi.
Competing Interest: None stated
|
What is Already Known?
• Monovalent hepatitis
B vaccine produces adequate immunity in term low birthweight
infants.
What This Study Adds?
•
Hepatitis B component of
pentavalent vaccine is equally immunogenic in term low
birthweight (1800-2499 g) infants as in normal weight babies.
|
References
1. Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC.
What level of hepatitis B antibody is protective? J Infect Dis.
1999;179:489-92.
2. Hollinger FB. Factors influencing the immune
response to hepatitis B vaccine, booster dose guidelines, and vaccine
protocol recommendations. Am J Med. 1989;87:36S-40S.
3. World Health Organization. Hepatitis B vaccines:
WHO position paper. Wkly Epidemiol Rec. 2009;84:405-20.
4. Lubchenco LO, Hansman C, Boyd E. Intrauterine
growth in length and head circumference as estimated from live births at
gestational ages from 26 to 42 weeks. Pediatrics. 1966;37:403-8.
5. Arora NK, Ganguly S, Agadi SN, Irshad M, Kohli R,
Deo M, et al. Hepatitis B immunization in low birthweight
infants: do they need an additional dose? Acta Paediatr.
2002;91:995-1001.
6. Bhave S, Bhise S, Chavan SC, Naik SS, Pusapati
RVLN, Bavdekar A, et al. Hepatitis B vaccination in premature and
low birth weight (LBW) babies. Indian Pediatr. 2002;39:625-31.
7. Sharma HJ, Yadav S, Lalwani SK, Kapre SV, Jadhav
SS, Chakravarty A, et al. Immunogenicity and safety of an
indigenously manufactured reconstituted pentavalent (DTwP-HBV+Hib)
vaccine in comparison with a foreign competitor following primary and
booster immunization in Indian children. Hum Vaccin. 2011;7:451-7.
8. Chatrath R, Saili A, Jain M, Dutta AK. Immune
status of full term small-for-gestational age neonates in India. J Trop
Pediatr. 1997;43:345-8.
9. Singh M, Manerikar S, Malaviya AN, Premawathi,
Gopalan R, Kumar R. Immune status of low birth weight babies. Indian
Pediatr. 1978;15:563-7.
10. Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G,
Yunus M, et al. Low birth weight is associated with altered
immune function in rural Bangladeshi children: a birth cohort study. Am
J Clin Nutr. 2007;85:845-52.
11. Ali SS, Chandrashekar SR, Singh M, Bansal RK,
Sharma DR, Arora D. A multicenter, prospective, open-label,
non-comparative study to evaluate the immunogenicity and tolerance of a
new, fully liquid pentavalent vaccine (DTwP-HepB-Hib vaccine). Hum
Vaccin. 2007;3:116-20.
12. Chatterjee S, Rego SJ, D’Souza F, Bhatia BD,
Collard A, Datta SK, et al. The immunogenicity and safety of a
reduced PRP-content DTPw-HBV/Hib vaccine when administered according to
the accelerated EPI schedule. BMC Infect Dis. 2010;10:298.
13. Gentile A, Umido V, Czerniuk P, Nacul J,
Seigelchifer M, Hilbert AK, et al. Immunogenicity and
reactogenicity of a combined fully liquid DTPw-HepB-Hib pentavalent
vaccine in healthy infants: No clinically relevant impact of a birth
dose of hepatitis B vaccine. Int J Infect Dis. 2011;15:24-9.
14. Sharma H, Yadav S, Lalwani S, Gupta V, Kapre S,
Jadhav S, et al. A phase III randomized, controlled study to
assess the immunogenicity and tolerability of DTPw-HBV-Hib, a liquid
pentavalent vaccine in Indian infants. Vaccine. 2011;29:2359-64.
15. Gatchalian S, Reyes M, Bernal N, Lefevre I, David
M, Han HH, et al. A new DTPw-HBV/Hib vaccine is immunogenic and
safe when administered according to the EPI (Expanded Programme for
Immunization) schedule and following hepatitis B vaccination at birth.
Hum Vaccin. 2005;1:198-203.
16. Gomber S, Sharma R, Ramachandran VG, Talwar V,
Singh B. Immunogenicity of hepatitis B vaccine incorporated into the
expanded program of immunization schedule. Indian Pediatr. 2000;37:411-3
17. Sharma H, Yadav S, Lalwani S, Kapre S, Jadhav S,
Parekh S, et al. Antibody persistence of two pentavalent DTwP-HB-Hib
vaccines to the age of 15-18 months, and response to the booster dose of
quadrivalent DTwP-Hib vaccine.Vaccine.2013;31:444-7.
18. Sadoh AE, Nwaneri DU, Ogboghodo BC, Sadoh WE.
Effect of introduction of pentavalent vaccine as replacement for
Diphtheria-Tetanus-Pertussis and Hepatitis B vaccines on vaccination
uptake in a health facility in Nigeria. Vaccine. 2016;34:2722-8.
|
|
|
 |
|

