|
|
|
Indian Pediatr 2017;54: 369-372 |
 |
Transcutaneous
Bilirubin Nomogram for Healthy Term and Late Preterm Neonates in
First 96 Hours of Life
|
|
Pareshkumar Thakkar, Hardas Chavda and *Vikas Doshi
From Departments of Pediatrics and *Community
Medicine, Medical College and SSG Hospital, Vadodara, Gujarat, India.
Correspondence to: Dr Pareshkumar Thakkar, 21, Jay
Gayatrinagar Society, Near Amitnagar, VIP Road, Vadodara 390 022,
Gujarat, India.
Email: [email protected]
Received: March 01, 2016;
Initial review: May 19, 2016;
Accepted: February 23, 2017.
Published online:
Match 29, 2017.
PII:
S097475591600049
|
Objective: To develop nomogram of Transcutaneous Bilirubin among
healthy term and late-preterm neonates during first 96 hours of age.
Design: Longitudinal observational study.
Setting: Neonatal unit of a tertiary care
Hospital of Central Gujarat, India.
Participants: 1075 healthy term and late preterm
neonates ( ³35weeks).
Intervention: Six-hourly transcutaneous bilirubin
was obtained from birth to 96 hour of life using Drager JM 103
Transcutaneous Bilirubinometer.
Main outcome measures: Nomogram of Transcutaneous
Bilirubin with percentile values was obtained, rate of rise of bilirubin
was calculated and predictive ability of normative data was analyzed for
subsequent need of phototherapy.
Results: The age-specific percentile curves and
nomogram were developed from the transcutaneous bilirubin readings of
1,010 neonates. Rate of rise in first 12 hour was 0.2 mg/dL and was 0.17
mg/dL in 12 to 24 hour of life which decreased on second day of life.
Neonates who required phototherapy had consistently higher readings of
transcutaneous bilirubin and also higher rate of rise in first 48 hrs.
Conclusion: Neonates whose transcutaneous
bilirubin is above the 50th percentile should be monitored for the
development of significant hyperbilirubinemia.
Keywords: Hyperbilirubinemia, Jaundice, Prognosis.
|
|
I
t is desirable that after delivery, newborn and
mother be discharged as early as possible but the risk of subsequent
development of neonatal hyperbilirubinemia is a major hurdle [1,2]. A
risk based approach has been advocated by AAP(American Academy of
Pediatrics) [3], and by NNF (National Neonatology Forum) of India [4].
However, significant hyperbilirubinemia can also occur in a neonate
without any identifiable risk factors. There is a need to have some
objective method which can reliably predict the subsequent development
of hyperbilirubinemia. The hour specific serum bilirubin nomogram by
Bhutani, et al. [5] is widely followed, but being invasive is
inconvenient for mass screening.
Transcutaneous bilirubin (TcB) measurement being a
non-invasive method is feasible for mass screening of at risk neonates.
Several studies have reported the utility of TcB as a surrogate of Total
Serum Bilirubin (TSB) [6]. TcB nomograms have been published from
several countries [7-9], but for Indian population, only one published
study is available [10]. The present study was carried out with the
objectives of developing TcB nomogram and to assess predictive ability
of these nomograms.
Methods
This was a prospective longitudinal study carried out
at Neonatal unit of Department of Pediatrics, Medical College Vadodara,
Gujarat over period of 6 months from December, 2013 to June, 2014. The
study was approved by scientific review committee and Institutional
ethics committee and waiver of consent was granted.
We included healthy term and late-preterm ( ³35
weeks) neonates. Exclusion criteria were babies with Rh-isoimmunization,
major malformation, hydrops fetalis, first encounter with patient after
1 hour of life, patients who left/discharged before 48 hours of lifes
and NICU admission for >6 hours. Babies who required phototherapy were
excluded for construction of nomogram. All newborns, whose mother were
Rh-negative or had positive Indirect Coombs test result, were evaluated
for blood group and Direct Coombs test results. Neonates who required
phototherapy were also evaluated for blood group, Direct Coombs test
results and G6PD deficiency.
TcB estimations were done by using Dragger JM – 103,
a hand held bilirubinometer that measures TcB levels by using
multiwavelength spectral reflectance analysis. All TcB measurements were
taken at forehead and we took average of five repeat measurements. All
readings were taken with same device by resident doctors, according to
the manufacturer’s instructions. TcB readings were taken every 6±1
hourly interval starting from 0 hour of life upto 96 hours. After 96
hours of life, whenever possible, we continued to take TcB readings, to
know normal change in bilirubin values. The need for phototherapy and
management of hyperbilirubinemia was ascertained by treating consultant
based on unit protocols and evidence-based practice guidelines of NNF
[4]. TSB estimation was done on clinical demand, when TcB values were
within 2 mg/dL or 80% of age specific threshold for starting
phototherapy (as per nomograms of Bhutani, et al. [5]) or when
value of TcB was >13 mg/dL.
Discharge and follow up plan was optimized and
individualized by a thorough pre-discharge assessment of risk factors
for severe jaundice. The institution has a discharge policy of not
discharging neonates before 48 hours of age. Neonates who did not return
for follow up were telephonically contacted on 14 th
day of life, to enquire whether the child needed any consultation or
admission for any morbidity including jaundice.
Statistical analysis: Baseline and outcome
data were recorded in a predesigned performa and master chart was
prepared in Microsoft Excel sheet. The data was entered into a
custom-designed interface in STATA-IC-13 software and checked for
completion, consistency and accuracy. TcB readings were clubbed in
6-hour epochs starting at 0 hour of age. Percentile for each epoch and
rate of rise in TcB level in different percentiles were obtained by
using same software.
Results
There were 1,782 neonates who were assessed for
eligibility; 1,075 neonates were finally included in the study,
of which 65 neonates who required phototherapy, were not included for
construction of nomogram (Fig. 1).
 |
|
Fig. 1 Study flow.
|
Of 1010 neonates, gender distribution was almost
equal, 28% had weight <2500 grams, 3.9% had gestational age of < 37
weeks and 6.1 % were SGA. All were exclusively breast fed and 969
(95.9%) mothers had received oxytocin during labour.
 |
|
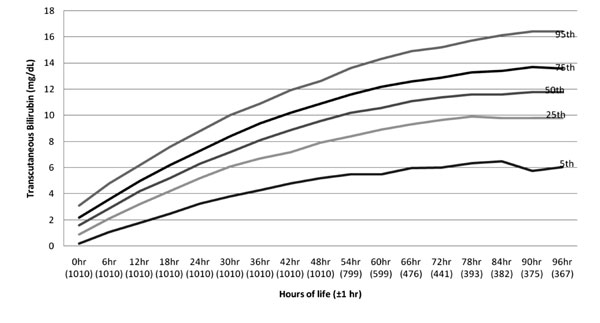
Fig. 2 Age-specific nomogram of
neonates up to 96 hours.
|
A total of 12,922 TcB measurements were available in
first 96 hours, nomogram of which is shown in Fig. 2. To
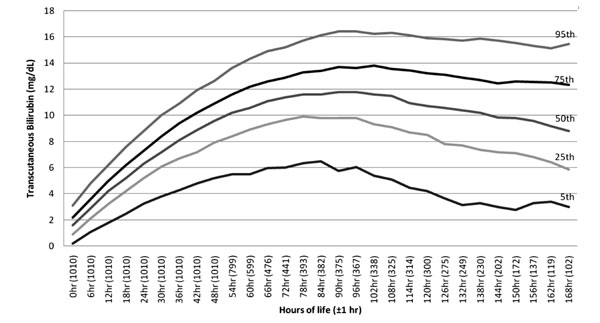
follow the normal pattern of bilirubin, 15685 reading were available
till 168 hours. Nomogram for this is shown in Fig. 3.
 |
|
Fig. 3 Age-specific nomogram of
neonates up to 168 hours.
|
|
Age in hrs |
TcB values at different
percentiles (mg/dL) |
|
5th |
25th |
50th |
75th |
95th |
|
0 |
0.2 |
0.9 |
1.6 |
2.2 |
3.1 |
|
6 |
1.1 |
2.1 |
2.9 |
3.6 |
4.8 |
|
12 |
1.8 |
3.2 |
4.2 |
5.0 |
6.2 |
|
18 |
2.5 |
4.2 |
5.2 |
6.2 |
7.6 |
|
24 |
3.2 |
5.2 |
6.3 |
7.3 |
8.8 |
|
36 |
4.3 |
6.7 |
8.1 |
9.4 |
10.9 |
|
48 |
5.2 |
7.9 |
9.6 |
10.9 |
12.6 |
|
60 |
5.5 |
8.9 |
10.6 |
12.2 |
14.3 |
|
72 |
6.0 |
9.6 |
11.4 |
12.9 |
15.2 |
|
96 |
6.0 |
9.8 |
11.8 |
13.6 |
16.4 |
|
TcB = transcutaneous bilirubin. |
TcB percentile values are depicted in Table
I. The mean value of TcB at 0 to 1 hour of age obtained was 1.6 mg/dL.
Peak value obtained was 11.6 mg/dL at 90 hours of age. The rate of rise
(ROR) of TcB percentiles is shown in Table II. The mean
value of ROR observed in first 12 hours of life was 0.2 mg/dL, 0.17 mg/dL
at 12 to 24 hours of life, 0.14 mg/dL and 0.12 mg/dL at 24-36 hours and
36 to 48 hours of life, respectively.
TABLE II Transcutaneous Biliruben Rate of Rise Percentiles for Normal Neonates (N=1010)
|
Age in hrs |
TcB rate of rise (mg/dL)
percentiles |
|
5th |
25th |
50th |
75th |
95th |
|
0-6 |
0.1 |
0.1 |
0.2 |
0.3 |
0.4 |
|
6-12 |
0.1 |
0.1 |
0.2 |
0.3 |
0.4 |
|
12-18 |
0.1 |
0.1 |
0.2 |
0.2 |
0.4 |
|
18-24 |
0 |
0.1 |
0.2 |
0.2 |
0.4 |
|
24-36 |
0 |
0.1 |
0.1 |
0.2 |
0.3 |
|
36-48 |
0 |
0.1 |
0.1 |
0.2 |
0.2 |
|
48-60 |
0 |
0.1 |
0.1 |
0.1 |
0.2 |
|
60-72 |
0 |
0.1 |
0.1 |
0.1 |
0.2 |
|
TcB = transcutaneous bilirubin. |
Total 65 (6.04%) neonates required phototherapy. The
TcB readings were consistently higher in neonates who required
phototherapy. The mean TcB value observed at 0-1 hour was 3.08 mg/dL
which is between 75 th
percentile to 95th
percentile of the nomogram and they cross a line of 95th
percentile at 54 hours with the mean TcB value of 13.76 mg/dL (Table
I).
The mean ROR observed in group who required
phototherapy was 0.22 mg/dL in first 12 hour compared to 0.2 mg/dL in
those who did not. The gap in ROR became wider in 12-24 hours and the
fall in ROR was at slower rate in neonates who required phototherapy.
So, predictive model based on ROR could be used for early discharge
policy if two TcB readings are obtained between 12-48 hours (Table
II). The 24hr 50 th
centile TcB’s predictive ability for phototherapy had a sensitivity and
negative predictive value of 100% and a specificity of 48.9%. Similarly
50th centile ROR of TcB
between 18-24 hour had a sensitivity of 83.1%, negative predictive value
of 97.7% and specificity of 47% to predict the need for phototherapy (Web
Table I).
Discussion
Present study provides normative data with various
percentile values of TcB and its rate of rise in term healthy and late
preterm Indian neonates. The neonates who required phototherapy had
consistently higher readings of TcB and ROR.
Nomograms using TcB have been developed by various
countries for their population, but only a single study from India by
Mishra, et al. [10]. The ROR observed in the study of Mishra,
et al. [10] is somewhat higher in first 48 hours compared to our
study. This may be because there were more pre-term neonates in their
study group compared to the present study. Maisels, et al. [7]
provide nomograms from a predominantly white population from North
America from a convenient sample of day time TcB measurements after six
hours of life. The overall TcB observations including all percentiles
are lower compared to our data. This can be due to almost all newborns
in our study were exclusively breastfed and due to racial and ethnic
variations. A systemic review by De Luca, et al. [11] had
compared four TcB nomogram developed in North America (mixed population)
[7], European [8], Hispanic [12] and Thai population [9]. The analysis
revealed significant differences in values of bilirubin across various
populations. Significant differences were observed in TcB values at
different percentiles at different hours of life, different rate of rise
and peak values of TcB. Nomogram have been also reported from Canada
[13,14], Brazil [15], China [16] and Israel [17] for healthy term and
late pre-term neonates. The Israel [17] study included only clinically
jaundiced neonates. The Brazilian [15] study included only term
neonates. Nomogram developed by De Luca, et al. [8] had also
included neonates who required phototherapy. Few of the authors have
given separate nomogram according to gestational age [7]. The major
limitation of almost all studies is that they are not population-based
and represent data from single center.
There were few limitations of this study. It is not a
population-based study; however, with increasing number of institutional
deliveries, our sample of healthy neonates by and large is
representative of normal neonatal population. For developing
country-wide nomogram, a multicenter trial should be conducted. The
nomogram should be validated for predictive ability with a separate
cohort of neonates.
The present study provides a nomogram of natural
history of bilirubin in healthy term and late pre-term neonates in
predominantly breast fed, unselected population. On the basis of this
data we conclude that neonates whose TcB is above the 50 th
percentile at 24 hrs should be closely monitored for development of
significant hyperbilirubinemia.
Contributors: PT: conceived and designed the
study; HC: was involved in data collection; PT, VD, HC: did analysis and
interpretation of data; PT, HC: drafted the manuscript. All authors have
approved the final version of manuscript.
Funding: None; Competing interest: None
stated.
|
What is Already Known?
• Transcutaneous Bilirubin estimation is
non-invasive and useful method for screening of neonates for the
risk of development of hyperbilirubinemia.
What This Study Adds?
• This study provides transcutaneous bilirubin nomogram for
healthy term and late preterm Indian neonates with percentiles
and its rate of rise.
|
References
1. Friedman MA, Spitzer AR. Discharge criteria for
the term newborn. Pediatr Clin North Am. 2004;51:599-618.
2. Maisels MJ, Kring E. Length of stay, jaundice, and
hospital readmission. Pediatrics. 1998;101:995-8.
3. Management of hyperbilirubinemia in the newborn
infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
4. Thakre R, Murki S, Venkataseshan S. Management of
Neonatal Hyperbilirubinemia. In: Evidence Based Clinical Practice
Guidelines. eds. Kumar P, Jain N, Thakre R, Murki S,
Venkatasestan. National Neonatology Forum, New Delhi; 2010: p. 139-54.
5. Bhutani VK, Johnson L, Sivieri EM. Predictive
ability of a predischarge hour-specific serum bilirubin for subsequent
significant hyperbilirubinemia in healthy term and near-term newborns.
Pediatrics. 1999;103:6-14.
6. Mahajan G, Kaushal RK, Sankhyan N, Sharma RL,
Nakra M. Transcutaneous bilirubinometer in assessment of neonatal
jaundice in northern India. Indian Pediatr. 2005;42:41-5.
7. Maisels MJ, Kring E. Transcutaneous bilirubin
levels in the first 96 hours in a normal newborn population of > or = 35
weeks’ gestation. Pediatrics. 2006;117:1169-73.
8. De Luca D, Romagnoli C, Tiberi E, Zuppa AA, Zecca
E. Skin bilirubin nomogram for the first 96 h of life in a European
normal healthy newborn population, obtained with multiwavelength
transcutaneous bilirubinometry. Acta Paediatrica. 2008;97:146-50.
9. Sanpavat S, Nuchprayoon I, Smathakanee C,
Hansuebsai R. Nomogram for prediction of the risk of neonatal
hyperbilirubinemia, using transcutaneous bilirubin. J Med Assoc Thai.
2005;88:1187-93.
10. Mishra S, Chawla D, Agarwal R, Deorari AK, Paul
VK. Transcutaneous bilirubin levels in healthy term and late preterm
Indian neonates. Indian J Pediatr. 2010;77:45-50.
11. De Luca D, Jackson GL, Tridente A, Carnielli VP,
Engle WD. Transcutaneous bilirubin nomograms: a systematic review of
population differences and analysis of bilirubin kinetics. Arch Pediatr
Adolesc Med. 2009;163:10.
12. Engle WD, Lai S, Ahmad N, Manning MD, Jackson GL.
An hour-specific nomogram for transcutaneous bilirubin values in term
and late preterm Hispanic neonates. Am J Perinatol. 2009;26:425-30.
13. Wainer S, Parmar SM, Allegro D, Rabi Y, Lyon ME.
Impact of a transcutaneous bilirubinometry program on resource
utilization and severe hyperbilirubinemia. Pediatrics. 2012;129:77-86.
14. Wainer S, Rabi Y, Parmar SM, Allegro D, Lyon M.
Impact of skin tone on the performance of a transcutaneous jaundice
meter. Acta Paediatrica. 2009;98:1909-15.
15. Draque CM, Sanudo A, de Araujo Peres C, de
Almeida MF. Transcutaneous bilirubin in exclusively breastfed healthy
term newborns upto 12 days of life. Pediatrics. 2011;128:e565-71.
16. Yu ZB, Dong XY, Han SP, Chen YL, Qiu YF, Sha L,
et al. Transcutaneous bilirubin nomogram for predicting neonatal
hyperbilirubinemia in healthy term and late-preterm Chinese infants. Eur
J Pediatr. 2011;170:185-91.
17. Bental YA, Shiff Y, Dorsht N, Litig E, Tuval L,
Mimouni FB. Bhutani-based nomograms for the prediction of significant
hyperbilirubinaemia using transcutaneous measurements of bilirubin. Acta
Paediatr.2009;98:1902-8.
18. Yu ZB, Han SP, Chen C. Bilirubin nomograms for
identification of neonatal hyperbilirubinemia in healthy term and
late-preterm infants: a systematic review and meta-analysis. World J
Pediatr. 2014;10:211-8.
|
|
|
 |
|

