|
|
|
Indian Pediatr 2017;54:
363-367 |
 |
Probiotics for Promoting Feed Tolerance in
Very Low Birth Weight Neonates ó A Randomized Controlled
Trial
|
|
A Shashidhar, PN Suman Rao, Saudamini Nesargi,
Swarnarekha Bhat and BS Chandrakala
From Department of Neonatology, St Johnís Medical
College, Bangalore, India.
Correspondence to: Dr Shashidhar A, Assistant
Professor, Department of Neonatology, St. Johnís Medical College,
Sarjapur Road, Bangalore 34, Karnataka, India.
Email: [email protected]
Received: June 09, 2016;
Initial review: October 14, 2016;
Accepted: February 11, 2017.
Published online:
March 29, 2017.
Trial Registration: CTRI/2012/08/002853.
PII: S097475591600053
|
Objective: To measure the efficacy of a probiotic formulation on
time to reach full enteral feeds in VLBW (very low birth weight)
newborns.
Design: Blinded randomized
control trial.
Setting: A tertiary care neonatal
intensive care unit (NICU) in Southern India between August 2012 to
November 2013.
Participants: 104 newborns
with a birth weight of 750-1499 g on enteral feeds.
Intervention: Probiotic group (n=52)
received a multicomponent probiotic formulation of Lactobacillus
acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum and
Saccharomyces boulardii once a day at a dose of 1.25◊109 CFU from
the time of initiation of enteral feeds till discharge and the control
group (n=52) received only breast milk.
Outcome measure: Time to reach
full enteral feeds (150 mL/kg/day).
Results: The mean (SD) time to
reach full enteral feeding was 11.2 (8.3) days in probiotic vs.
12.7 (8.9) in no probiotic group; (P=0.4), and was not
significantly different between the two study groups. There was a trend
towards lower necrotizing enterocolitis in the probiotic group (4% vs.
12%).
Conclusion: Probiotic
supplementation does not seem to result in significant improvement of
feed tolerance in VLBW newborns.
Keywords: Bifidobacterium, Infant feeding,
Lactobacillus, Necrotizing entrocolitis.
|
|
Enteral feeding intolerance is
a major issue in
premature infants, resulting in prolonged
hospitalization and a predisposition to serious
complications due to prolonged use of parenteral nutrition. A delay in
reaching full enteral feedings is also associated with a poorer mental
outcome in preterm neonates at 24 months corrected age [1].
As the maturation of motor activity in premature
infants lags behind that of digestive and absorptive functions, it is
most frequently a disorder of gastrointestinal motility that limits the
use of enteral feeding in this population. An adequate establishment of
the intestinal flora after birth is strictly related to motility
maturation and plays a crucial role in the development of gut barrier
function and the innate and adaptive immune system [2].
Enteral supplementation of probiotics prevents severe
necrotizing enterocolitis (NEC) and all-cause mortality in preterm
infants [3]. Moreover, among the many strategies tried for prevention of
feeding intolerance, probiotics are the most promising. However, they
are yet to be used as standard of care. The objective of this trial was
to determine the efficacy of probiotics on feed tolerance in very low
birthweight (VLBW) neonates.
Methods
All neonates with a birth weight between 750 g to
1499 g admitted to the NICU in whom enteral feeds were started were
eligible for enrolment. Neonates with gastro-intestinal anomalies,
severe congenital malformation, and those not started on enteral feeds
by day 14 of life were excluded. The study was a double blind randomized
controlled trial (RCT) conducted in a tertiary care NICU between
August 2012 to November 2013.
We hypothesized that by establishing a normal
intestinal flora probiotics could reduce the incidence of feed
intolerance. The study protocol was approved by the Institutional Ethics
Committee and a written informed consent was taken from the
parent/guardian before enrollment.
Outcomes: The primary outcome of the study
was the time taken to reach full enteral feeds. Secondary outcomes were
episodes of feed intolerance, incidence of NEC stage 2 or more,
duration of hospital stay, days on total parenteral nutrition (TPN),
weight gain and mortality during hospital stay.
Feed intolerance was defined as presence of any one
of the following four features - abdominal distension
≥2 cm from the
previous measurement; or vomiting ≥2
episodes in the past 6 hours or blood stained or bilious; or gastric
aspirate >2 episodes of voluminous gastric aspirates in a 6 hr period.
Voluminous gastric residuals were defined as >50% of previous feed
volume if ≥6
mL/feed; or 2 episodes of >50% in a 6 hr period or single residue of
100% if feed volume <6 mL/feed.
Sample size: The sample size was determined based
on data from a pilot study done in the same clinical unit. To achieve a
significant mean difference of 3.37 days (with SD 5.05 and 6.6 in the 2
groups) in time to reach full feeds with a Type 1 error of 5% and a
Power of 80% (2 sided), the sample size required was 47 (in each group)
which was inflated by a further 10% based on the usual percentage of
deaths and discharges against medical advice in the VLBW group in our
Unit.
The subjects were randomly allocated into two groups
using computer generated random numbers by an investigator not directly
involved in the study. We followed a parallel group design and block
randomization was done with block sizes varying from 8 to 12.
Sequentially numbered opaque sealed envelopes were used for allocation
concealment. The two groups were coded as A and B and the group code was
kept off site in an opaque sealed envelope and opened only after the
final analysis was done.
Feeding protocol: Feeding was initiated,
advanced, stopped and restarted as per unit protocol derived by
consensus for the purpose of the study. The protocol was attached to all
the study case files to ensure compliance. Trophic feeds i.e 10
to 20 mL/kg/day at 2 hourly interval of either colostrum (if available)
or donor breast milk feeds were initiated in hemodynamically stable
infants. Feeds were advanced by 20 mL/kg/day (in babies 750-1249 g and
those with abnormal antenatal Doppler) or by 35mL/kg/day (in babies
1250-1499g and well). Feeds were given every 2 hourly and pre-feed
aspirates were measured in babies on gavage feeds. Feeds were withheld
if there were signs of feed intolerance, hemodynamic instability,
suspected NEC or voluminous gastric residuals. Feeds were restarted when
all the above mentioned signs were resolved. Parenteral nutrition was
continued till 100 mL/kg/day of feeds were reached. Full feeds were
defined as 150 mL/kg/day. Oral feeds were initiated in babies more than
30-32 weeks with good suck reflex and otherwise well. 5% Dextrose was
used if milk was not available. Human milk fortifiers were used as per
NNF India recommendations.
The weights were checked daily on a calibrated
digital weighing machine with a sensitivity of Ī5 g. A best gestational
age was given for each infant based on LMP and corroborated by early
first trimester ultrasound when available. NEC was defined and staged as
per modified Bellís staging. The infants were discharged as per unit
protocol which is baby maintaining hemodynamic stability without
support, on full oral feeds-either by breast milk or paladay, showing
consistent weight gain of 15 to 20 g/kg/day for 3 days and 1.4 kg or
more. The management protocols, clinical practices, equipment,
infrastructure, and key personnel were unchanged during the study
period. The baseline illness severity documented by Score for Neonatal
Acute Physiology Perinatal extension (SNAPPE-II) was used in both groups
[4]. Adequate antenatal steroids were defined as an interval of at least
24 hrs after the initial dose.
Probiotic group received a multicomponent probiotic
formulation of Lactobacillus acidophilus, Lactobacillus rhamnosus,
Bifidobacterium longum and Saccharomyces boulardii in the
form of powdered sachets of 1g each (Darolac; Aristo Pharmaceuticals
Pvt. Ltd.). The intervention was administered once a day at a dose of
1.25◊10 9 CFU starting within
24 hours of initiation of feeds. It was given as a powder form dissolved
in breast milk and from day 2, given at a fixed time of the day. Fresh
suspensions of supplements were individually prepared in the pantry
under strict asepsis by study nurses who were not directly involved in
routine patient care for each study infant. The probiotic
supplementation was continued till discharge given once a day if the
volume of feeds was 2 mL or more, and in two divided doses if the baby
received <2 mL/feed. It was stopped when feeds were withheld for any
reason. The no probiotic group received only breast milk and served as
the control. No placebo was used.
The probiotic supplementation did not change the
physical appearance of the milk, and the syringes were labeled only with
the patientís name and identification number with no indication of study
group assignment. Attending physicians and nurses caring for the infants
were blinded to the group assignments. To ensure blinding, the mixing
was done after the milk required for each study infant was gathered away
from the patient care area in the NICU irrespective of the assigned
group. The probiotic was stored as per manufacturerís guidelines and was
prescribed to all infants enrolled in the study to ensure blinding.
Nurses followed strict asepsis during preparation and
compliance was monitored regularly by one of the investigators. The
infants were clinically monitored daily by the consultants for feed
intolerance and sepsis. A septic screen followed by blood culture was
done as per clinical suspicion.
Statistical analysis: Continuous variables were
compared by using Studentís t test or the Mann-Whitney U test when
appropriate; chi square analysis or Fisherís exact test when
appropriate, was used to ascertain significant differences in
categorical variables between groups. All tests were 2-tailed.
Significance was defined as P<0.05. Intention to treat analysis of data
was performed. The final statistical analysis was performed using SPSS
20.0 software (SPSS Inc, Chicago, IL, USA)
Results
Of the 162 VLBW babies admitted to the NICU during
the study period, 104 VLBW newborns were enrolled; 52 in each group.
Fig. 1 shows the flow of study subjects through the phases of
the study.
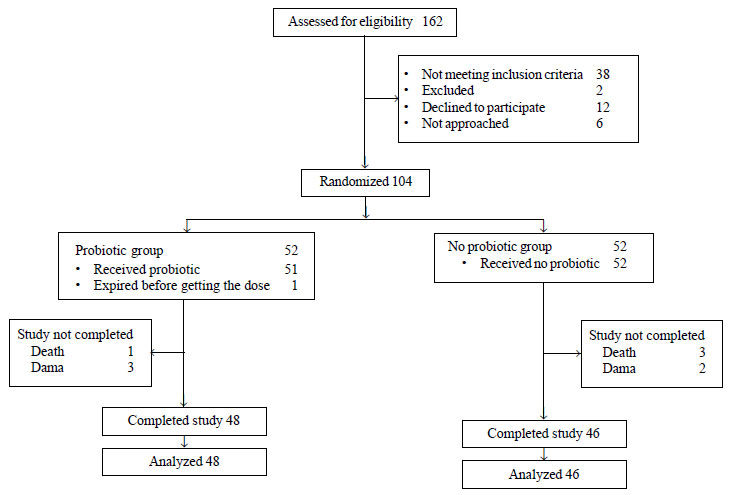 |
|
Fig. 1 Trial flow.
|
The baseline characteristics are depicted in
Table I. The groups were comparable except for slightly more
number of Caesarean deliveries in the no probiotic group (Table
I). There were 5 extremely preterm babies (4 in control and 1 in
probiotic group) and 15 extremely low birth weight babies (10 in control
and 5 in probiotic group). Enteral feeding was initiated at a similar
postnatal age in the probiotic and no probiotic groups. Oral
supplementation with probiotics began in parallel with enteral feeding
(<24 h after initiation of feeds). A mean duration of 26.3 (17.6) days
of probiotic supplementation was received in the intervention group. 22
babies in the probiotic group and 25 in the control group were
exclusively fed with breast milk.
TABLE I Baseline Characteristics of Participants
|
Characteristics |
Probiotic (n=52) |
No probiotic (n=52) |
|
Gestational age *(wk) |
31.2 (2.1) |
31 (2.1) |
|
Sex (M:F) |
27:25 |
20:32 |
|
Birth weight *(g) |
1256 (185) |
1190 (208) |
|
Out born, n (%) |
10 (19.2) |
8 (15.3) |
|
Primigravida, n (%) |
30 (57.6) |
31 (59.6) |
|
Small for gestation, n (%) |
18 (34.6) |
19 (36.5) |
|
Gestational hypertension, n (%) |
21 (40.3) |
31 (59.6) |
|
Abnormal doppler, n (%) |
12 (23) |
9 (17.3) |
|
SNAPPE score* |
6.7 (7.9) |
8.3 (9.9) |
|
Caesarean delivery, n (%) |
27 (51.9) |
38 (73) |
|
Adequate antenatal steroids, n (%) |
27 (51.9) |
27 (51.9) |
|
APGAR at 1 min* |
6.6 (2.6) {n= 45} |
6.7 (1.5) {n= 46} |
|
APGAR at 5 min* |
8 (0.8) {n= 45} |
8 (1.0) {n= 46} |
|
Age at initiation of enteral feeds# (hrs.) |
15 (6,51) |
17 (9,47.5) |
|
*Mean (SD); #Median (IQR). |
The primary outcome of time to full enteral feeding
was 11.2 (8.3) days in probiotic vs 12.7 (8.9) in no probiotic
group and was not significantly different (Table II). The
secondary outcomes were also comparable between the groups. Treatments
such as duration of ventilation and antibiotic usage, and other co
morbidities like intraventricular hemorrhage, patent ductus arteriosus,
respiratory distress syndrome and bronchopulmonary dysplasia were not
significantly different between the two groups.
TABLE II Outcomes in Preterms in Probiotic and Control Groups
|
Parameter |
Probiotic group |
No probiotic group |
Mean difference
|
P
|
|
(n=48) |
(n=48) |
(95% CI) |
value |
|
Time to reach full feeds in days1 |
11.2 (8.3) |
12.7 (8.9) |
-1.5 (-4.9,1.9)
|
0.4 |
|
Duration of hospital stay (days)* |
27.6 (18.5) |
31.2 (22.9) |
-3.6 (-11.7,4.5) |
0.4 |
|
Duration of total parenteral nutrition (days)* |
9.5 (8.3) |
10.5 (9) |
-1.0 (-4.4,2.4) |
0.5 |
|
Number of episodes of feed intolerance# |
1 (0,2) |
1(0,2) |
0 (0.7,0.7) |
1.0 |
|
Number of withheld feeds# |
21 (1,40.5) |
12 (0,48) |
2.7 (-14.7,20) |
0.8 |
|
Weight gain per week (g)* |
31.1(27) |
39.5 (32.3) |
-8.4 (-20,3.2) |
0.2 |
|
Necrotizing enterocolitis ≥stage
II (%) |
2 (4.1) |
6 (12.5) |
3 (0.6,14.2)$ |
0.3 |
|
Mortality, n (%) |
1(1.9) |
3 (5.7) |
3 (0.3,27.9)$ |
0.6 |
|
*Mean(SD); #Median (IQR); $Relative risk
(95% CI). |
No unexpected adverse events were observed during the
course of the study. There was no significant difference in the
incidence of nosocomial infection, including fungal sepsis, in the
probiotic group.
Discussion
The present RCT comparing use of probiotic versus
no probiotic did not observe any significant reduction in the time
to reach full enteral feeding. However, VLBW infants receiving
probiotics reached full feeds 1.5 days earlier, which was similar to the
result of recently published systematic review [9].
The strength of the study was the blinding used. We
did not evaluate the successful colonization of infantsí gut by the
organism. The sample size derived from our pilot study was probably
over-optimistic on the expected effect size, as the difference observed
was much smaller.
The results of our study were similar to other
studies [6-12]. The RCTs which have shown a positive effect on time to
full feeds are the ones from India [13] and France [6]. However, the
Indian study enrolled infants at 5 days and allocation concealment, and
blinding of intervention and outcome was not adequately described [14],
and the French study [6]
showed a benefit only in >1000 g babies. In our
RCT, feeds were initiated at a median of 15 and 17 hours, respectively,
in the probiotic and no probiotic group which was comparatively early
compared to other RCTs which report a mean of about 3 to 5 days. Though
many trials and the Cochrane review [14] have shown a favorable impact
on time to reach full feeds with probiotics, i.e. three days
earlier than the control group (95% CI: 2.78 to 3.69 days, P<0.001),
many recent trials cited earlier have shown no improvement. The
guidelines for use of probiotics have been published [15] a few months
after initiation of our study. However, on review, our methodology was
consistent with most of the recommendations. There could be several
explanations for the observed results. It could be because of
predominant use of human breast milk in our NICU which is a rich natural
source of probiotic organisms and protects against NEC [16]. Most
studies showing benefit in NEC have had a significant use of non-human
milk. The second reason could be cross-contamination resulting in
nosocomial acquisition of probiotic strains by the other group in the
unit as evidenced by Kitajima [17] resulting in narrowing of differences
between the two groups. Cross-contamination in the control arm is
expected to underestimate the true effects of probiotics. A
significantly higher number of caesarean deliveries in the no probiotic
group (52% vs 73%) could have narrowed the differences. The
intestinal flora of caesarean delivered infants is altered and
characterized by a substantial absence of Bifidobacteria sp., and
vaginally delivered is characterized by predominant groups such as B.
longum and B. catenulatum. Therefore, the infants who would
have probably benefited more by probiotics were in the control arm. It
may also be due to the different strains used in our study which were
chosen on the basis of availability and existing literature of the time.
In conclusion, in the present double-blind randomized
trial, oral supplementation with multicomponent probiotic formulation of
L. acidophilus, L. rhamnosus, B.longum and S. boulardii
did not improve the gastrointestinal tolerance to enteral feeding in
very-low-birth weight infants, but was a safe intervention. We suggest
larger multicentric trials of probiotic supplementation to achieve early
feed tolerance before accepting/rejecting probiotics for wider clinical
use.
Acknowledgements: Nursing staff of the St
Johnís Medical College NICU for their invaluable help in this research,
parents of the infants who took part in this study, Dr.Tinku Sarah for
helping with the statistical analysis.
Contributorsí: SA: conceptualized and
designed the study, drafted the initial manuscript, and approved the
final manuscript as submitted; SR, SB: reviewed and revised the
manuscript, and approved the final manuscript as submitted; SN, CBS:
designed the data collection instruments, and coordinated and supervised
data collection.
Funding: None; Competing interest: None
stated.
References
1. Morris BH, Miller-Loncar CL, Landry SH, Smith KE,
Swank PR, Denson SE. Feeding, medical factors, and developmental outcome
in premature infants. Clin Pediatr (Phila). 1999;38:451-7.
2. Indrio F, Riezzo G, Cavallo L, Mauro A Di,
Francavilla R. Physiological basis of food intolerance in VLBW. J Matern
Neonatal Med. 2011;24:64-6.
3. Patole S. Nutrition for the Preterm Neonate - A
Clinical Perspective. Springer, Nether Lands; 2013.
4. Richardson DK, Corcoran JD, Escobar GJ, Lee SK.
SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality
risk scores. J Pediatr. 2001;138:92-100.
5. Athalye-Jape G, Deshpande G, Rao S, Patole S.
Benefits of probiotics on enteral nutrition in preterm neonates: a
systematic review. Am J Clin Nutr. 2014;100:1508-19.
6. Rouge C, Piloquet H, Butel MJ, Berger B, Rochat F,
Ferraris L, et al. Oral supplementation with probiotics in
very-low-birth-weight preterm infants: A randomized, double-blind,
placebo-controlled trial. Am J Clin Nutr. 2009;89:1828-35.
7. Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J,
Pohlandt F. Effect of Bifidobacterium lactis on the incidence of
nosocomial infections in very-low-birth-weight infants: a randomized
controlled trial. Neonatology. 2010;98:156-63.
8. Manzoni P, Mostert M, Leonessa ML, Priolo C,
Farina D, Monetti C, et al. Oral supplementation with
Lactobacillus casei subspecies rhamnosus prevents enteric colonization
by Candida species in preterm neonates: A randomized study. Clin Infect
Dis. 2006;42:1735-42.
9. Lin H-C, Hsu C-H, Chen H-L, Chung M-Y, Hsu J-F,
Lien R, et al. Oral probiotics prevent necrotizing enterocolitis
in very low birth weight preterm infants: a multicenter, randomized,
controlled trial. Pediatrics. 2008;122: 693-700.
10. Fernandez-Carrocera LA Cabanillas-Ayon M,
Gallardo-Sarmiento RB, Garcia-Perez CS, Montano-Rodriguez R, Echaniz-Aviles
MO S-HA. Double-blind, randomised clinical assay to evaluate the
efficacy of probiotics in preterm newborns weighing less than 1500 g in
the prevention of necrotising enterocolitis. Arch Dis Child. Fetal
Neonatal Ed. 2013;98:F5-9.
11. Braga TD, da Silva GAP, de Lira PIC, de Carvalho
Lima M. Efficacy of Bifidobacterium breve and Lactobacillus casei oral
supplementation on necrotizing enterocolitis in very-low-birth-weight
preterm infants: a double-blind, randomized, controlled trial. Am J Clin
Nutr. 2011;93:81-6.
12. Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M,
Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing
enterocolitis in very low birth weight neonates. J Pediatr.
2005;147:192-6.
13. Samanta M, Sarkar M, Ghosh P, Ghosh J kr, Sinha
MK, Chatterjee S. Prophylactic probiotics for prevention of necrotizing
enterocolitis in very low birth weight newborns. J Trop Pediatr.
2009;55:128-31.
14. Alfaleh K, Anabrees J, Bassler D, Al-Kharfi T.
Probiotics for prevention of necrotizing enterocolitis in preterm
infants. Cochrane Database Syst Rev. 2011;3:CD005496.
15. Deshpande GC, Rao SC, Keil AD, Patole SK.
Evidence-based guidelines for use of probiotics in preterm neonates. BMC
Med . 2011;9:92.
16. Lucas A., Cole TJ. Breast milk and neonatal
necrotising enterocolitis. Lancet. 1990;336:1519-23.
17. Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama
H, Fujimura M. Early administration of Bifidobacterium breve to preterm
infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed.
1997;76:F101-7.
|
|
|
 |
|

