|
|
|
Indian Pediatr 2016;53:
391-393 |
 |
Neurodevelopmental
Outcome of Extremely Low Birth Weight Children at
Corrected Age of Two Years
|
|
Kanya Mukhopadhyay, Rama Mahajan, Prahbhjot Malhi and
Ashok Kumar
From Neonatal Unit and Child Psychology Unit,
Department of Pediatrics, PGIMER, Chandigarh, India.
Correspondence to: Dr Kanya Mukhopadhyay, Professor,
Neonatology, Department of Pediatrics, PGIMER,
Chandigarh 160 012, India.
Email: [email protected]
Received: July 06, 2015;
Initial review: August 20, 2015;
Accepted: March 08, 2016.
|
Objective: To assess the neurodevelopmental, cognitive and
behavioral function of extremely low birth weight babies (ELBW) till
corrected age of two years.
Methods: 79 ELBW babies were enrolled and
followed at 1 year (n=50), 18 months (n=47) and 2 years (n=36).
Adverse composite outcome was defined as death or moderate-to- severe
neurodevelopmental impairment (defined as either cerebral palsy or DQ
score <70 or deafness or blindness).
Results: At 1 year, 24% were neurologically
abnormal. At 18 months, average score (>85) was seen in 25 (54%)
children in motor and 8 (17%) in mental development. Abnormal behavioral
score ( ³12)
was seen in 89% children. Adverse composite outcome was present in 28
(35.4 %) babies.
Conclusion: ELBW neonates are at a high risk of
neurodevelopmental and behavioral impairment.
Keywords: Behavior disorders, Cerebral Palsy, Neurological
disorders, Preterm, Prognosis.
|
|
Availability of advanced neonatal care has led to
increasing survival of extremely low birth weight (ELBW) babies. Several
studies have reported adverse long term neuro-developmental outcome of
these infants [1-3]. However, there is paucity of data from developing
nations on outcome of these infants. In developing countries including
India, a high proportion of ELBW babies are small for gestational age
(SGA). Outcome of this population may not be comparable to reports from
the developed world. Accurate knowledge of outcomes would be helpful in
parental counseling and decision- making in our resource-limited
scenario. We report the neurodevelopmental outcome in a cohort of ELBW
graduates from a NICU of a tertiary-care center.
Methods
All consecutive ELBW neonates born between January
2009 and March 2011 in our center and discharged alive were
prospectively followed up till corrected age (CA) of 2 years for their
neurodevelopmental and behavioral outcome. Institute research ethics
committee approved the study and informed consent was obtained from the
parents. They were followed up 3-monthly till CA 1 year and then 4-6
monthly till CA 2 years. At each visit, developmental screening was done
by Denver Developmental Screening Test (Denver II) (DDST) [4] and
neurological status assessed by Amiel-Tison scale [5] by the consultant
neonatologist. DDST was interpreted as ‘normal’ or ‘suspect’ as per the
manual. Neurological examination was categorized as either abnormal (hypertonia
or hypotonia) or normal. Babies with hypertonia were labeled as spastic
Cerebral Palsy (CP). Developmental Assessment Scale for Indian Infants
(DASII) [6] was conducted by a trained neonatologist at CA 18 months.
DASII scores <70, 70- 85 and >85 were defined as delay, borderline and
average, respectively. Behavior was assessed by Preschool Behavior Check
List (PBCL) [7] at CA 2 years, and a score of
³12 was considered
high. Moderate to severe neurodevelop-mental impairment (NDI) was
defined as either CP, or DQ <70 in DASII scale, or blindness, or
deafness requiring hearing aids. Adverse composite outcome included the
above or death. Mild NDI was defined as mild hypotonia or DQ between
70-85 [8]. Statistical analysis was done using SPSS version 18.
Quantitative variables are reported as mean (SD) and qualitative
variables as proportions. Comparison was made using student t test or
chi square test, as appropriate.
Results
Of the 255 live births during the study period, 36
babies were followed till 2 years of age (Fig. 1).
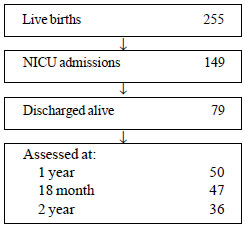 |
|
Fig. 1 Study flow chart.
|
The mean (SD) birth weight and gestation of the
babies who were discharged alive were 874 (81) g and gestation 29.9
(2.2) weeks, respectively and 48 were SGA (62.3%).
At CA 1 year, 38 (76%) were neurologically normal and
12 (24%) were abnormal (7 hypotonic and 5 hypertonic). According to
Denver II assessment, 36 (72%) were normal and 14 (28%) were suspect.
Adverse composite outcome (moderate to severe NDI including death) was
diagnosed in 35.4% (28) at CA 18 months. In followed up cases (n=57),
adverse composite outcome was seen in 49% (28) and in 38% (18, if death
excluded). Minor NDI was diagnosed in 45.6% (26). Only 3 children were
normal at corrected age 18 months. Mean (SD) of MoDQ, MeDQ and PBCL were
83.1 (16.1), 72.7 (16.1) and 19.4 (4.1), respectively. Thirty two (89%)
had high PBCL (score ³12).
TABLE I Motor and Mental Development Quotient in ELBW Children at Corrected Age of 18 Months (N=47)
|
Development Quotient |
MoDQ, n (%) |
MeDQ, n (%) |
|
<70 |
12(25%) |
15(32%) |
|
70-85 |
10(21%) |
24(51%) |
|
>85 |
25(54%) |
8(17%) |
|
Values are expressed as n (%); Mo and Me are motor and mental
DQ, respectively. |
Table I depicts the mental (MeDQ) and motor
developmental quotients (MoDQ) and PBCL and their subcategories. Overall
MeDQ score was lower than MoDQ scores. We did not find any significant
effect of any risk factor on the adverse composite outcome (Table
II).
TABLE II Risk Factors for Death or Neurodevelopmental Impairment (NDI) in ELBW Babies
|
Variables |
Death and Moderate |
No (n=3) and Mild |
OR (95% CI) |
P value |
|
to severe NDI (n=28), No. (%) |
NDI (n=26), No. (%) |
|
|
|
Ventilated |
14 (50) |
13 (45) |
1.2 (0.4-3.4) |
0.78 |
|
Male |
18 (64) |
13 (45) |
2.1 (0.7-6.5) |
0.17 |
|
Small for gestational age |
14 (50) |
8 (27.5) |
0.5 (0.2-1.6) |
0.27 |
|
Culture positive sepsis |
12 (43) |
10 (34) |
0.7 (0.2-2.2) |
0.59 |
|
Apnea |
15 (53.5) |
13 (45) |
1.3 (0.5-3.9) |
0.55 |
|
Hypoglycemia |
8 (28.5) |
6 (21) |
1.5 (0.4-5.1) |
0.53 |
|
Abnormal USG head (IVH/PVL) |
10 (36) |
13 (45) |
0.6 (0.2-1.9) |
0.41 |
|
Bronchopulmonary dysplasia |
8 (28.5) |
9 (31) |
0.8 (0.25-2.51) |
0.70 |
|
IVH: Intraventricular haemorrhage; PVL:
Periventricular leucomalacia. |
Discussion
We assessed an ELBW cohort by using three different
tests to detect neurodevelopmental impairment. Neurological abnormality
was seen in 24%, Denver II was suspect in 28% and delay (DQ < 70) was in
25% and 32% in MoDQ and MeDQ, respectively.
The major limitation of our study was a very small
sample size due to lower rates of survival of ELBW babies [9,10] and
limited duration of study. Hence it was difficult to calculate
associations of various risk factors to abnormal outcome. Another
limitation was high drop-out rate as patients came from very far-off
places.
Most of the outcome studies of ELBW are reported in
less than 25-26 weeks of gestation [1,11]; however, the mean gestation
of our babies was ~29 weeks and proportion of growth retarded babies was
higher. Rates of severe NDI has been reported as 17-59% at 18-36 months
in ELBW babies [1,11] and gestation is an important variable in
calculating risk of NDI. Our higher NDI rates in spite of higher
gestation probably can be explained due to high sepsis rate, high SGA
proportion and poor postnatal growth [10].
Cerebral palsy was diagnosed in 3.7% and low DQ
(70-85) was seen in one-third of babies in a South African study with
mean gestation of 30 weeks and birth weight of 1182 grams [12]. A
similar rate of NDI was reported by NICHD nearly 2 decades ago in babies
with gestational age of 27-32 weeks, in which NDI ranged from 28-40% and
only 21% ELBW babies were reported as normal [8]. The Epicure study
reported severe impairment in 13.4% and moderate impairment in 11.8% and
CP in 14%. Risk factors of CP were male sex, intrauterine growth
restriction, surgery, postnatal steroids and high frequency ventilation
[2].
Our Mean DQ was comparable to NICHD reports of a mean
of 76 [3] and varied from center to center between 70-83 [13]. However,
their mean gestation was 26 (2) weeks as opposed to our higher mean
gestation. Similar to our data, mean DQ of <70 was seen in 23-30% babies
in a cohort of babies who were less than 32 weeks of gestation [8]. High
rates of behavioral abnormalities is also a well-recognized feature of
ELBW babies, which was also observed by us [14].
We had nearly 30% drop out at 18 months CA. Most of
the follow-up studies report a dropout rate of 5-30% [11], and a high
dropout rate attributes to a biased higher NDI due to higher reporting
of NDI in followed-up cases than non-followed up cases and this issue
has been highlighted in a systematic review [15].
With high rates of NDI in ELBW babies, early
intervention facilities must be developed across all neonatal intensive
care units in our country. For greater generalizability, a multicentric
study with a large number of cases and at least 80- 90% follow up rate
is desirable.
Contributors: KM: conceptualized and designed the
study, analyzed data and drafted the manuscript; RM: collected the data
and helped in data analysis; PM: supervised cognitive and behavioral
assessments; AK: conducted behavioral assessments.
Funding: PGI Research scheme; Competing
interests: None stated.
|
What This Study Adds?
• Extremely low birth weight babies are at a
high risk of neurodevelopmental and behavioral abnormalities;
Mental development is affected more than motor development in
these babies.
|
References
1. Jarjour IT. Neurodevelopmental outcome after
extreme prematurity: A review of the literature. Pediatr Neurol.
2015;52:143-52.
2. Moore T, Hennessy EM, Myles J, Johnson SJ, Draper
ES, Costeloe KL, et al. Neurological and developmental outcome in
extremely preterm children born in England in 1995 and 2006: the Epicure
studies. BMJ. 2012;345:e7961.
3. Vohr BR, Wright LL, Dusick AM, Mele L, Verter J,
Steichen JJ, et al. Neurodevelopmental and functional outcomes of
extremely low birth weight infants in the National Institute of Child
Health and Human Development Neonatal Research Network, 1993-1994.
Pediatrics. 2000;105:1216-26.
4. Frankenburg WK, Dodds J, Archer P, Shapiro H,
Bresnick B. The Denver II: A major revision and restandardization of the
Denver Developmental Screening Test. Pediatrics. 1992;89:91-7.
5. Amiel-Tison C. Update of the Amiel-Tison
neurologic assessment for the term neonate or at 40 weeks corrected age.
Pediatr Neurol. 2002; 27:196-212.
6. Misra N, Pathak P. Developmental Assessment Scales
for Indian Infants (DASII): Manual. Baroda, MS University of Baroda,
1996.
7. McGuire J, Richman N. Screening for behavior
problems in nurseries. The reliability and validity of the Pre- School
Behavior Checklist. J Child Psychol Psychiatry. 1986;27:7-32.
8. Vohr BR, Wright LL, Poole WK, McDonald SA.
Neurodevelopmental outcomes of extremely low birth weight infants <32
weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116:635-43.
9. Mukhopadhyay K, Louis D, Mahajan R, Kumar P.
Predictors of mortality and major morbidities in extremely low birth
weight neonates. Indian Pediatr. 2013;50: 1119-23.
10. Mukhopadhyay K, Louis D, Mahajan G, Mahajan R.
Longitudinal growth and post discharge mortality and morbidity among
extremely low birth weight neonates. Indian Pediatr. 2014;51:723-6.
11. Wilson-Costello D, Friedman H, Minich N, Siner B,
Taylor G, Schluchter M, et al. Improved neurodevelopmental
outcomes for extremely low birth weight infants in 2000-2002.
Pediatrics. 2007;119: 37-45.
12. Ballot DE, Potterton J, Chirwa T, Hilburn N,
Cooper PA. Developmental outcome of very low birth weight infants in a
developing country. BMC Pediatr. 2012;12:11.
13. Vohr BR, Wright LL, Dusick AM, Perritt R, Poole
WK, Tyson JE, et al. Neonatal Research Network. Center
differences and outcomes of extremely low birth weight infants.
Pediatrics. 2004;113:781-9.
14. Stephens BE, Vohr BR. Neurodevelopmental outcome
of the premature infant. Pediatr Clin North Am. 2009;56: 631-46.
15. Guillén U, DeMauro S, Ma L, Zupancic J, Roberts
R, Schmidt B, et al. Relationship between attrition and
neurodevelopmental impairment rates in extremely preterm infants at 18
to 24 months: A systematic review. Arch Pediatr Adolesc Med.
2012;166:178-84.
|
|
|
 |
|

