|
|
|
Indian Pediatr 2014;51:
397-398 |
 |
Langerhans Cell Histiocytosis Presenting as
Isolated Mediastinal Mass in an Infant
|
|
Mohammed Ramzan and Satya Prakash Yadav
From Pediatric Hematology Oncology and BMT Unit,
Department of Pediatrics, Fortis Memorial Research Institute, Gurgaon,
Haryana, India. .
Correspondence to: Dr Satya P Yadav, Department of
Pediatrics, Fortis Memorial Research Institute,
Gurgaon, Haryana, India.
Email: [email protected]
Received: July 26, 2013;
Initial review: October 04, 2013;
Accepted: February 05, 2014.
|
|
Background: Isolated mediastinal involvement in Langerhans cell
histiocytosis (LCH) has been rarely reported. Case characteristics:
A 3-month-old boy presented with history of low grade intermittent
fever, cough and noisy breathing for 2 weeks. Observation: A
chest X-ray showed massive mediastinal widening. Biopsy of the
mass confirmed LCH. Outcome: Patient is doing well after one year
of treatment with LCH III protocol. Message: Langerhans cell
histiocytosis should be considered in differential diagnosis of
mediastinal mass in infants.
Keywords: Cancer chemotherapy, Histiocytosis,
Mediastinal widening.
|
|
Langerhans cell histiocytosis (LCH) is a rare
disease characterized by monoclonal proliferation of dendritic-cell
related histiocytes. It frequently involves bones, skin, hypothalamus
and "risk organs" (liver, lung, spleen, hematopoietic system) [1]. We
report a rare presentation of LCH as an isolated mediastinal mass.
Case Report
A 3-month-old boy (full-term, birth weight 3.0 kg)
presented with history of low grade intermittent fever along with cough
and noisy breathing for two weeks. On examination, child had respiratory
distress with crepitation heard on auscultation over chest. There was no
hepato-splenomegaly, rash or ecchymosis. Blood examination and other
laboratory studies were normal. He needed supplemental oxygen to
maintain normal oxygen saturations and received a trial of nebulized
salbutamol with no apparent benefit. A chest X-ray showed massive
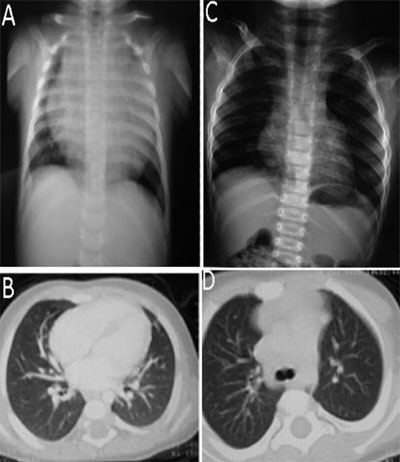
mediastinal widening (Fig.1) and computerized tomography
(CT) scan (Fig.1) of the chest showed a large lobulated
heterogeneous soft tissue mass (15x15 cm) in the anterior mediastinum
with some stromal enhancement and tracheal compression but no osteolysis.
Bone marrow aspiration, serum alpha-feto-protein and beta HCG levels
were normal.
 |
|
Fig. 1 Chest X-ray and CT thorax showing large
mediastinal mass pre-chemotherapy (A, B) and good response post-
chemotherapy (C, D).
|
A CT-guided biopsy of the mass was done;
histopathology showed medium sized, coffee bean shaped hyperchromatic
cells with abundant pinkish cytoplasm and heavy eosinophilic
infiltration. Immunohistochemical stains were positive for S-100 and
CD1a. A diagnosis of LCH was made. Skeletal survey, including whole body
bone scans (Tc-99m) did not identify any malignant focus.LCH III
chemotherapeutic regimen [2] was adopted for treatment. Induction phase
consisted of weekly injection of vinblastine (6 mg/m 2)
and oral prednisolone (40 mg/m2)
for 6 weeks. After induction chemotherapy, noisy breathing and other
chest symptoms improved significantly and CT chest showed reduction of
mediastinal mass by 50%. He received another 6 weeks of re-induction
chemotherapy. After 12 weeks, a follow-up CT scan of the chest showed
significant reduction in the size of the mediastinal mass with minimal
residual lesion (Fig.1). He was started on maintenance
therapy (vinblastine injection once every 3 weeks, oral 6-mercaptopurine
50 mg/m2 daily and
prednisolone orally 40 mg/m2
for 5 days once every 3 weeks) for a total duration of 1 year. At
present child is off treatment for one year and is doing well without
any symptoms or sign of original disease.
Discussion
Our case illustrates that mediastinal compression due
to LCH in a young infant can be due to present as an isolated
mediastinal mass. Germ cell tumor, thymic hyperplasia, congenital cysts,
lymphoma, intrathoracic thyroid tissue and lymphangioma are the common
anterior mediastinal mass in this pediatric age group [3]. LCH must be
included in the differential diagnosis of such lesions as early
diagnosis is key to successful therapy. Though absent in our case,
typical seborrheic involvement of the scalp may be mistaken for
prolonged "cradle cap" in infants. Infants may also present with skin
involvement as brown to purplish papules over any part of their body,
and they should be followed up regularly [4]. Punctuate or serpentine
calcification/cysts are usual radiographic features of lung LCH, but
these were absent in our case. Confluence of cysts may lead to bullous
formation and spontaneous pneumothorax can be the first sign of LCH in
the lung [5]. Though many cases have been reported to present as
mediastinal mass along with multisystemic involvement, isolated
mediastinal LCH has been reported rarely [7-10]. Recently, a French LCH
registry [6] enrolled 1426 patients with LCH in last 20 years; 37 (2.6%)
had mediastinal mass, and majority were infants.
Emergent and adequate treatment for sufficient
duration is necessary in infant LCH as it may be fatal. Higher rate of
reactivation and a higher mortality rate has been reported in
mediastinal mass group as compared to non-mediastinal mass group. In LCH
III trial, the overall 5-year survival of risk organ positive (RO+)
patients was 84% that was higher than in the corresponding (historical)
RO+ patients in the predecessor LCH-I (62%) and LCH-II (69%) trials.
Underscoring the importance of treatment duration on reactivation
frequency, LCH-III RO+ patients (12-month treatment) had a 27% 5-year
risk of reactivation, much lower than that of the comparable (RO+)
historical controls treated in LCH-I (55%) and LCH-II (44%), who
received only 6 months of therapy [2]. This clearly shows the importance
of prolongation of treatment in LCH patients.
We conclude that mediastinal LCH should be considered
in differential diagnosis of mediastinal mass in infants. Timely
diagnosis and treatment can lead to good outcome.
Contributors: Both authors contributed equally to
the mauscript.
Funding: None; Competing interests: None.
References
1. Howarth DM, Gilchrist GS, Mullan BP, Wiseman GA,
Edmonson JH, Schomberg PJ. Langerhans’ cell histiocytosis: diagnosis,
natural history, management, and outcome. Cancer. 1999;85:2278-90.
2. Gadner H, Minkov M, Grois N, Pötschger U, Thiem E,
Aricò M, et al. Therapy prolongation improves outcome in
multisystem langerhans cell histiocytosis. Blood. 2013;121:5006-14.
3. Duwe BV, Sterman DH, Musani AI. Tumors of the
mediastinum. Chest. 2005;128: 2893.
4. Munn S, Chu AC. Langerhans cell histiocytosis of
the skin. Hematol Oncol Clin North Am. 1998;12:269-86.
5. Bernstrand C, Cederlund K, Henter JI. Pulmonary
function testing and pulmonary langerhans cell histiocytosis. Pediatr
Blood Cancer. 2007;49:323-8.
6. Ducassou S, Seyrig F, Thomas C, Lambilliotte A,
Berard PM, Berger C, et al. Thymus and mediastinal node
involvement in childhood langerhans cell histiocytosis: long-term
follow-up from the french national cohort. Pediatr Blood Cancer.
2013;60:1759-65. 7.
7. Mogul M, Hartman G, Donaldson S, Celb A, Link M,
Amylon M, et al. Langerhans cell histiocytosis presenting with
the superior vena cava syndrome: A case report. Med Pediatr Oncol.
1993;21:456-9.
8. Elliott M, Kokai GK, Abernethy LJ, Pizer BL.
Spontaneous resolution of isolated thymic Langerhans cell histiocytosis.
Med Pediatr Oncol. 2002;38:274-6.
9. Hernandez Perez JM, Franquet CT, Rodriguez S,
Giminez A. The langerhans cell histiocytosis with thymic localization as
initial and exclusive place. Ann Med Interna. 2007;24:497-9.
10. Khadilkar UN, Rao ATK, Sahoo KK, Pai MR.
Langerhans cell histiocytosis of mediastinal node. Indian J Pediatr.
2008;75:294-6.
|
|
|
 |
|

