|
Achieving optimal enteral feeding is crucial for
the growth of the newborn, and delay in establishing full enteral feed
is associated with adverse short term and long term outcomes [1].
Advancement of enteral nutrition in preterm neonates is hampered by
gastric residuals and feeding intolerance. Gastrointestinal hypomotility
and non-development of coordinated peristalsis are the postulated
reasons for feeding intolerance [2-5].
Various feeding strategies (like trophic feeding and
slow feeding advancement), medications (like antenatal steroids and
prokinetics), and meconium evacuation are used to improve feeding
intolerance [6-9]. Immature intestinal motor mechanism and
neurotransmitter system is considered to be responsible for delay in
passage of meconium in preterm infants. Retained thick tenacious
meconium leads to functional obstruction, abdominal distension, gastric
residuals and feeding intolerance [10-12]; and total meconium evacuation
may improve feeding volume [11,13,14]. We hypothesized that glycerin
suppository – by acting as osmotic laxative – will facilitate early
meconium evacuation and accelerate feed tolerance and advancement in
preterm very-low-birthweight (VLBW) neonates. Our specific objective was
to compare the efficacy of glycerin suppository versus no
glycerin suppository in preterm VLBW neonates on time required to
achieve full enteral feeds.
Methods
This randomized controlled trial was conducted in a
Level III neonatal unit in Mumbai, India from March 2010 to April 2011.
The study was approved by the hospital’s Academic research ethics
committee. Infants were enrolled after obtaining written informed
consent from parents or legal guardians.
Infants admitted to neonatal intensive care unikt
(NICU) on day-1 of life, with a birthweight between 1000 to 1500 grams,
and gestational age between 28 to 32 weeks were eligible for inclusion
in the study. Infants with gastrointestinal or other systemic
malformations were excluded. Infant with hemodynamic instability and
features of shock were also excluded.
Eligible neonates were randomized to either glycerin
suppository group or control group. Those in the glycerin suppository
group received the drug at a dose of one infant glycerin suppository (Hallens
Infant Glycerin Suppository 1g, Meridian
Enterprises) once a day from day-2 of life onwards. Those assigned to
control group were not given any suppository, only a sham procedure was
performed (no active placebo). Sequence was generated using random
allocation software in variable blocks of 2 or 4, by a statistician who
was not part of the study. Randomization allocation concealment was
achieved by creating sequentially numbered sealed opaque envelopes that
contained the randomization codes. When a patient meeting inclusion
criteria was admitted in the unit, doctor on duty obtained written
informed consent from parents. The serially numbered opaque sealed
envelope was opened by the designated study staff nurses. Access to
these envelopes was only limited to these designated study staff nurses.
Nursing staff involved in care of infant, on-duty resident doctors and
consultants involved in the care of infant were blinded to study
intervention. The glycerin suppository administration or sham procedure
was performed by designated study staff nurses (two) behind curtains.
This intervention was continued till day 14 of life irrespective of
passage of stools. Glycerin suppository or sham intervention was
withheld if there was hemodynamic instability. These two study nurses
were otherwise not involved in day-to-day clinical management of these
infants. Feeds were started in both groups of infants when they were
clinically stable, usually between 3rd
to 5th day of life. The
feeds were in the form of intermittent boluses every 2 hours through
orogastric infant feeding tube. All infants received either expressed
breast milk (EBM) and/or preterm infant milk formula (Lactodex LBW,
Raptakos, Brett and Co. Ltd.); EBM was preferred, if available. The
initial feeding volume was 20 mL/kg/day and the volume was increased
daily by 20 mL/kg/day, if tolerated until complete enteral feeding was
achieved (180 mL/Kg/day). Feeds were withheld if clinical signs and
symptoms suggestive of necrotizing enterocolitis (NEC) or other
intra-abdominal pathology were suspected. Feeding was withheld as per
clinical condition or if gastric residuals exceeded 20% of previous feed
volume. EBM was fortified with a commercial powder preparation (Lactodex
HMF, Raptakos, Brett and Co. Ltd) once full feeds were tolerated by
infant. Feeding policy was similar in both study groups.
Standards of care of infants in the NICU did not
change throughout the study period. Grading up of intravenous fluids,
parenteral nutrition and starting of trophic feeds was done as before as
per the standard protocol of the unit. The regimen of parenteral
nutrition used for both groups remained same and it consisted of
solution of 10% dextrose, amino acids and electrolytes. Parenteral
nutrition was started on day-2 of life in all infants and advanced daily
to meet each infant’s total estimated fluid and nutritional
requirements.
The primary outcome was time required by the infant
to achieve full enteral feeds. (when the infant tolerated a volume of
180 mL/kg/day for at least 24 hours). Secondary outcomes were: time
required to regain birth weight, age of achieving a weight of 1700
grams, necrotizing enterocolitis (Bell classification) [15], proportion
of infants in whom feeds were withheld for any reasons, and age at the
time of discharge from hospital. All infants were followed up till the
time of discharge from the hospital or transfer to other hospital. All
the data were recorded in predesigned study case record forms.
Sample size was calculated by using the formula for
the hypothesis of 2-parallel sample means. In our unit, with the
existing feeding practices, the average time taken by an infant with
birth weight of 1000 to 1500 gram to reach full feeds was 15 days (SD 3
days). We hypothesized that the glycerin suppository group will reach
full feeds by day 12 of life. For a difference of 3 days, with an error
of 0.05 and power 90%, the estimated sample size was 22 in each group.
To account for loss to follow up, 25 infants were to be enrolled in each
group.
Data were analyzed by using IBM SPSS version 18
software. Categorical variables were compared using the Fisher’s exact
test. Continuous measures between groups were compared using two sample
t test or Mann-Whitney U test as appropriate. P value
<0.05 was considered significant.
Results
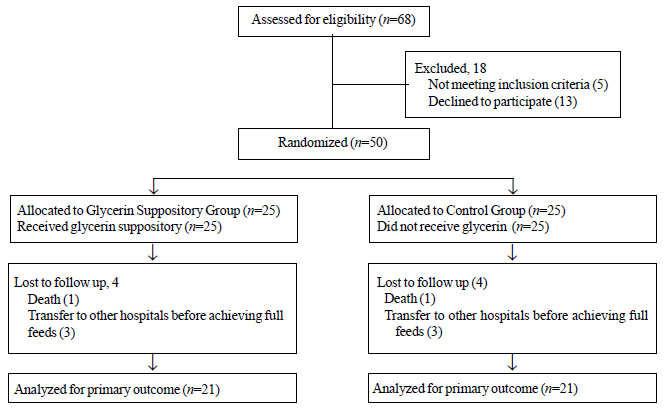
The flow chart of participants in the study is
presented in Fig. 1. A total of 50 infants were
randomized; 25 to glycerin suppository group and 25 to control group.
 |
|
Fig.1 Flow of participants in the
study.
|
The baseline clinical and demographic
characteristics of the two study groups were similar (Table I).
Table II depicts the outcomes in the two study groups. The
time required to reach full enteral feeds was similar in both groups.
Glycerin suppository group regained birth weight 2 days earlier than
control group but this difference was not statistically significant. The
age of achieving a weight of 1700 grams, proportion of infants with NEC,
proportion of infants in whom feeds were withheld, and age at discharge
from hospital were similar in both study groups.
TABLE I Baseline Demographic and Clinical Characteristics of The Study Participants
|
Characteristic |
Glycerin |
Control
|
|
Suppository
|
Group |
|
Group (n=25) |
(n=25) |
|
Birth weight (g)* |
1179 (142) |
1237 (161) |
|
Gestational age (wks)* |
29.7 (1.3) |
29.7 (1.1) |
|
Males, n (%) |
13 (52%) |
16 (64%) |
|
Antenatal glucocorticoids |
19 (76%) |
20 (80%) |
|
LSCS delivery |
16 (64%) |
14 (56%) |
|
Age at introduction of feeds (d)*
|
5.5 (2.2) |
5.5 (2.8) |
|
Type of milk received |
|
Breast milk only |
18 (72%) |
19 (76%) |
|
Breast milk+Formula milk |
7 (28%) |
6 (24%) |
|
* Values in mean (SD). |
TABLE II Comparison of Outcomes in Glycerine Suppository vs. Control Groups
|
* Outcome
|
Glycerin suppository |
Control group |
Relative risk (95% CI) |
|
group (n=21) |
( n=21) |
|
|
Time to full enteral feeds (d)
|
11.9 (3.1) |
11.3 (3.6) |
0.57 (-1.52 to 2.66) |
|
Time to regain birth weight (d)
|
15.7 (4.1) |
17.1 (1.3) |
-1.45 (-3.35 to 0.45) |
|
Age of achieving weight of 1700 g (d)
|
41.6 (9.7) |
36.3 (8.8) |
5.32 (-0.46 to 11.10) |
|
NEC stage 2 or more, n |
0/21 |
1/21 |
0.33 (0.01 to 7.74) |
|
NEC stage 1, n |
2/21 |
0/21 |
5.0 (0.25 to 98.27) |
|
Feeds withheld, n |
7/21 |
4/21 |
1.75 (0.60 to 5.10) |
|
Duration of hospital stay (d)
|
53.7 (14.4) |
48.9 (14.8) |
4.8 (-4.31 to 13.91) |
|
* Values in mean (SD). |
Discussion
In this randomized controlled trial on 50 VLBW
neonates, we could not detect any difference in time required by the
infant to achieve full enteral feeds in children receiving glycerin
suppository and those not receiving it.
We did not enroll infants <1000 grams in our study
because of perceived risk and difficulty in administering 1g infant
glycerin suppository in this population. The main limitations of our
study was the assessment of only short term outcomes. Moreover, our
study was not powered for outcomes like NEC. We calculated sample size
for 3 days reduction in time required to reach full enteral feeds. A
smaller reduction that may still be clinically meaningful may have been
missed as it requires a large sample size.
Shim, et al. [4], in an observational study,
reported a significant reduction in time to achieve full enteral feeds.
A recent randomized controlled trial by Khadr, et al. [1] showed
that the median time to full feeds was 1.6 days shorter in glycerin
suppository group; however, it was not statistically significant. Haiden,
et al. [6] used gastrografin osmotic contrast for evacuation of
meconium in preterm infants, and observed that the median time to reach
full enteral feedings was shorter in intervention group as compared to
control group. Two systematic reviews evaluating glycerin suppository
for meconium evacuation did not report any benefit on feeding
intolerance or hyperbilirubinemia [16,17]. It may be possible that
glycerin application every 24 hour until complete achievement of full
feeds may be too low to be efficient. A more frequent application (e.g.12
hourly) or higher dose may be more effective in accelerating meconium
evacuation [18]. It is also possible that meconium evacuation cannot be
accelerated by suppositories because rapid and sufficient meconium
passage indicates a correct functionality of the digestive tract.
IIn conclusion, once daily application of glycerin
suppository does not seem to offer any advantage in premature infants in
terms of accelerating achievement of full enteral feeding.
Contributors/i>: SS: review of literature, data
collection and wrote the first draft; NSK: management of patients, study
design, drafting the article, analysis and interpretation of data. He
will act as guarantor; SRS, BSA, JA: management of patients, designing
of study and drafting the manuscript. The final manuscript was approved
by all the authors.
Funding : None; Competing interests:
None stated.
|
What is Already Known?
• Glycerin suppository is useful in early
evacuation of meconium in term and preterm infants.
What This Study Adds?
• DDaily application of glycerin
suppository in preterm infants (gestational age 28-32 weeks)
does not have any advantage in accelerating full enteral
feeding.
|
References
1. Khadr SN, Ibhanesebhor SE, Rennix C, Fisher HE,
Manjunatha CM, Young D, et al. Randomized controlled trial:
impact of glycerin suppositories on time to full feed in preterm
infants. Neonatology. 2011;100:169-76.
2. Leaf A. Introducing enteral feeds in the high-risk
preterm infant. Semin Fetal Neonatal Med. 2013:18;150-4.
3. Bisset WM, Watt J, Rivers RP, Milla PJ.
Postprandial motor responses of the small intestine to enteral feeds in
preterm infants. Arch Dis Child. 1989;64:1356–61.
4. Shim SY, Kim HS, Kim DH, Kim EK, Son DW, Kim BI,
et al. Induction of early meconium evacuation promotes feeding
tolerance in very low birthweight infants. Neonatology. 2007;92:67-72.
5. McClure RJ, Newell SJ. Randomised controlled trial
of trophic feeding and gut motility. Arch Dis Child Fetal Neonatal Ed.
1999;80:54-8.
6. Haiden N, Norooz F, Klebermass-Schrehof K, Horak
AS, Jilma B, Berger A, et al. The effect of an osmotic contrast
agent on complete meconium evacuation in preterm infants. Pediatrics.
2012;130:e1600-6.
7. Patole S. Strategies for prevention of feed
intolerance in preterm neonates: a systematic review. J Matern Fetal
Neonatal Med. 2005;18: 67-76.
8. McGuire W, Henderson G, Fowlie PW. Feeding the
preterm infant. BMJ 2004;329: 1227-30.
9. Costalos C, Gounaris A, Sevastiadou S,
Hatzistamatiou Z, Theodoraki M, Alexiou EN, et al/i>.. The effect of
antenatal corticosteroids on gut peptides of preterm infants – a matched
group comparison: corticosteroids and gut development. Early Hum Dev.
2003;74:83-8.
10. Yamauchi K, Kubota A, Usui N, Yonekura T, Kosumi
T, Nogami T, et al. Benign transient non organic ileus of
neonate: Eur J Peditr Surg. 2002;12:168-74.
11. Seigel M. Gastric emptying time in premature and
compromised infants. J Pediatr Gastroenterol Nutr. 1983:2 Suppl 1;
s236-40.
12. Dimmitt RA, Moss RL. Meconium diseases in infants
with very low birth weight. Semin Pediatr Surg. 2000;9:79-83.
13. Mihatsch WA, Franz AR, Linder W, Pohlandt F.
Meconium passage in extremely low birth weight infants and its
relationship to very early enteral nutrition. Acta Paediatr.
2001;90:409-11.
14. Neu J, Zhang L. Feeding intolerance in very
low-birth weight infants: what is it and what can we do about it? Acta
Paediatr. 2005;94:Suppl.93-9.
15. Bell MJ, Ternberg JL, Feigin RD, Keating JP,
Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis.
Therapeutic decisions based upon clinical staging. Ann Surg. 1978;
187:1-7.
16. Shah V, Chirinian N, Lee S. EPIQ evidence review
Group; Does the use of glycerin laxatives decrease feeding intolerance
in preterm infants? Paediatr Child Health 2011;16:e68-70.
17. Shrinivasjois R, Sharma A, Shah P, Kava M. Effect
of induction of meconium evacuation using per rectal laxative on
neonatal hyperbilirubinemia in term newborn; A systemic review of
randomized controlled trials. Indian J Med Sci. 2010;65:278-85.
18. Emil S, Nguyen T, Sills J, Padilla G. Meconium
obstruction in extremely low-birth weight neonates: guidelines for
diagnosis and management. J Pediatr Surg; 2004; 39:731-7.
|

