|
|
|
Indian Pediatr 2012;49:
371-376 |
 |
Survival After Immunosuppressive Therapy in
Children with Aplastic Anemia
|
|
Velu Nair, *Vishal
Sondhi, $Ajay Sharma,
$Satyaranjan Das and
$Sanjeevan Sharma
From the Department of Medicine, Armed Forces Medical
College, Pune, Maharashtra; †Department of Pediatrics,
Military Hospital, Ambala Cantt, Haryana and $Department of Hematology
and Bone Marrow Transplantation,
Army Hospital (Research and Referral Centre), New Delhi, India.
Correspondence to: Dr Velu Nair, Professor and Head of
Department, Department of Medicine, Armed Forces Medical College, Pune,
Maharashtra, India.
Email: [email protected]
Received: April 6, 2011;
Initial review: May 2, 2011;
Accepted: June 21, 2011.
Published online: 2011 October 30.
P II: S09747559110000298 – 1
|
Objective: To
determine the survival of children Ł18y,
treated with immunosuppresive therapy (IST) using equine antithymocyte
globulin (e-ATG) and cyclosporine (CsA).
Design: Prospective data entry as per a specified
format.
Setting: Tertiary care hospital.
Patients: From January 1998 to December 2009, 40
children were diagnosed with acquired aplastic anemia; 33 patients, who
received IST, were analyzed. 31 children (94%) received one course of
e-ATG and CsA. 2 patients (6%) received two courses of ATG.
Intervention: Immunosuppressive therapy using
equine ATG and cyclosporine.
Main Outcome Measures: Overall response and
overall survival.
Results: The overall response (complete response
+ partial response) to IST at 6 months was 87.9%. 8 (24.2%) patients
achieved CR, 21 (63.6%) patients had PR and 4 (12.1%) patients did not
respond to IST. Median follow-up was 24 (6-102) months. Overall survival
at 24 months was 90%, with an acturial survival of 85.4% at 5 years.
Seventeen patients (51.5%) received G-CSF for a median duration of 32
(23-64) days. The patients who received G-CSF had fewer infectious
complications (P=0.002), but G-CSF administration did not
influence survival/ outcome. No patient developed myelodysplastic
syndrome or acute leukemia.
Conclusions: The survival of patients who respond
to IST is excellent. Also, G-CSF reduces the infectious complications
without conferring any survival advantage.
Key words: Antithymocyte globulin, Aplastic anemia,
Ciclosporine, Granulocyte-Colony Stimulating Factor (G-CSF),
Immunosuppressive therapy, India, Treatment.
|
|
A plastic anemia is a
bone-marrow failure disorder characterized by immune mediated bone
marrow destruction, and immunosuppressive therapy forms an essential
aspect of therapy [1]. In general, the outcome after
hematopoietic-stem-cell-transplantation has been found to be better than
immuno-suppressive therapy, but since most children lack a
histocompatible donor, it is often administered as the initial therapy.
The standard regimen includes anti-thymocyte globulin (ATG) plus
cyclosporine (CsA) [2-4].
Several independent studies have predicted survival
ranging from 67.5% to 80% [5-8]. Furthermore, though the role of
Granulocyte-Colony Stimulating Factor (G-CSF) addition to
immunosuppressive therapy is debatable, many centers use additional
G-CSF, particularly in pediatric patients [8-10]. To gain insights into
the survival of children treated with immune suppressive therapy, we
conducted a single center analysis of overall response and overall
survival in children with aplastic anemia treated with ATG plus CsA.
Methods
All patients ≤18y
of age, diagnosed as aplastic anemia at a tertiary care center in India,
from January 1998 to December 2009 were included in the study. Patients
were excluded if they were diagnosed with an inherited marrow failure
syndrome before treatment or if they underwent stem cell transplant. The
details regarding medical history, physical examination, complete blood
count, bone marrow aspirate and biopsy were retrieved. The inherited
bone marrow failure syndromes were excluded based on medical history,
family history, physical examination, bone marrow cytogenetics, and
chromosomal fragility studies with diepoxybutane, echocardiogram and
ultrasound of the abdomen. Paroxysmal Nocturnal Hemoglobinuria (PNH) was
excluded by Hams’s test and urine for hemosiderin (till 2004), and by
flow cytometry for determination of CD55 and CD59 from January 2005
onwards. Additional tests including liver function tests, renal function
tests, and serology for hepatitis A,B,C, Epstein-Barr virus (EBV),
cytomegalovirus (CMV), and parvovirus B-19 were performed depending upon
clinical setting.
In addition, the data regarding the therapeutic
profile of the patients’ therapy with ATG and CsA, supportive therapy
with antibiotics, transfusions and G-CSF was obtained. All the courses
of ATG and CsA were documented and response to therapy at 6 month, 12
month, 18 month and last follow-up was recorded. The data was entered as
per a pre-specified proforma.
Disease severity: Patients were classified
according to published severity criteria [11,12]. Aplastic anemia was
considered severe if the marrow cellularity was <25%, and at least 2 of
the following criteria were met: neutrophil count <0.5×10 9/L,
platelet count <20×109/L, or
reticulocyte count <20×109/L.
It was considered very severe if the above criteria were fulfilled, and
the neutrophil count was <0.2×109/L.
Moderate aplastic anemia was defined as hypocellular bone marrow with at
least two of the following hematological values: neutrophil count <1×109/L,
platelet count <50×109/L, or
reticulocyte count <60×109/L,
but not sufficient for severe category. Hepatitis-associated aplastic
anemia was defined when it occurred either concurrent or within 6 months
after presentation with an increase in serum alanine amino- transferase
level by at least five times the upper reference limit.
Treatment protocol: The treatment was initiated
only after obtaining consent from the parents of the child. Equine ATG
(e-ATG, Atgam, Pfizer Inc, New York, NY) was administered intravenously
at a dose of 40 mg/kg/day for 4 days as continuous infusion over 12-18h.
On day 1, it was administered at a very slow rate intravenously
initially to evaluate for immediate hypersensitivity reaction, and if
there was no reaction to infusion, it was infused at the regular rate
for next 12-18h. CsA was administered orally from day 21 of ATG at a
dose of 8-10 mg/kg/day in 2 divided doses and adjusted to maintain serum
levels between 150-200 µg/L or for renal/hepatic toxicity.
Oral prednisolone at a dose of 2 mg/kg/day was
administered for 7 days followed by a 1 week taper for prevention of
serum sickness. Platelets were transfused prophylactically for levels
<10×10 3/L and at higher
levels in setting of symptomatic bleeding. Red blood cell transfusions
were given for hemoglobin <70g/L or symptomatic anemia. All blood
products were irradiated [12]. Most patients received single donor
platelet units. However, random donor platelets were also used. Febrile
neutropenia was managed with intravenous antibiotics with addition of
antifungals after 3-4 days of unresponsive fever in accordance with the
institutional antimicrobial policy. No prophylactic oral antifungals or
antibiotics were administered. G-CSF was administered at a dose of
5µg/kg/day subcutaneously. The point of initiating G-CSF was not
predefined and was variable in different patients. G-CSF was used in all
patients of very severe aplastic anemia, and was not used in patients in
moderate category. In patients with, G-CSF was administered if the
patient had febrile neutropenia or sepsis, based on the treating
physicians discretion. A second course of e-ATG or rabbit ATG (r-ATG,
Thymoglobulin, Genzyme Corporation, Cambridge, MA) was administered, if
the patient had not responded after 6 months of initial treatment or
relapsed after initial response. CsA was administered for 12 months and
was, thereafter, tapered gradually over next 3-6 months, so that each
patient received CsA for 15-18 months.
Response criteria: A complete response was
defined as neutrophils >1.5×10 9/L,
platelets >100×109/L and
hemoglobin value normal for age and sex. A partial response was defined
when the counts were not sufficient for a complete response and the
absolute neutrophil count (ANC) was >0.5×109/L,
platelets >20×109/L and
hemoglobin >80 g/L in patients with severe, and ANC >1.0×109/L
and platelets >30×109/L and
hemoglobin >80 g/L in patients with moderate aplastic anemia. The
response was assessed 6 months after sATG administration. Relapse was
indicated by a decline in peripheral blood cell counts to levels meeting
the definition of severe or moderate aplastic anemia.
For detecting clonal disorders, the patients were
followed up using peripheral blood counts and biochemistry. The bone
marrow and cytogenetic studies were attempted only if the blood counts
or biochemical profile showed any abnormality.
Statistical analysis: Overall response was
calculated as the sum of partial and complete response. Overall survival
(OS) was measured from the time of onset of treatment to the time of
last follow-up or death. Summary statistics, including means, medians,
and proportions were used to describe patients’ baseline
characteristics. The multi-variate Cox regression model was used to
analyze the risk factors for death. Variables with P values <0.1
in univariate analysis were entered in stepwise selection models and
hazard ratios (HR) with 95% confidence intervals (CI) were calculated.
Survival analysis was done using the Kaplan-Meier curves. Statistical
analysis was done using GraphPad Prism version 5.00 for MacOsX (GraphPad
Software, San Diego California USA) and SPSS version 16.0 (SPSS Inc,
Ill, USA).
Results
From January 1998 to December 2009, 40 children were
diagnosed as acquired aplastic anemia; 7 were excluded (one died on day
2 of ATG administration and six did not consent for receiving IST). In
total, 33 patients who received IST were included for final analysis (Table
I). No PNH positive cases were detected during the study.
TABLE I Pretreatment Characteristics of Study Population (N=33)
|
Variable |
|
Age (yr)* |
14 (7y-18y) |
|
M:F |
1.54:1 |
|
Duration of symptoms* |
2.5 (1-15) mo |
|
Fever, n (%) |
16/33 (48%) |
|
Bleeding diathesis, n (%) |
22/33 (66.7%) |
|
Pallor, n (%) |
23/33 (70%) |
|
Hemoglobin (g/dL)* |
6.9 (3.5-9.3) |
|
Absolute reticulocyte count (X109/L)* |
19 (14-22) |
|
WBC count (X109/L)* |
1.6 (0.85-3.35) |
|
ANC count (X109/L)* |
0.35 (0.145-0.820) |
|
Platelets (X109/L)* |
14 (4-28) |
|
*Values in median (range). |
Of the 33 patients, one had hepatitis-associated-
aplastic anemia; the serological tests for hepatitis A, B, C, CMV, and
EBV were negative. No other causes for secondary aplastic anemia were
found in any other patient. Twenty-eight patients had idiopathic severe
aplastic anemia, 4 had very severe (3 idiopathic, one hepatitis
associated), and one child had idiopathic moderate aplastic anemia.
Response to immunosuppressive therapy
Thirty-one children (94%) received one course of
e-ATG. Two patients (6%) received two courses of ATG. One child who
failed to respond to first course of e-ATG was administered a second
course of e-ATG, but he continued to be a non-responder. The other
patient received r-ATG as the second course for relapse, after first
course of e-ATG and he responded to the therapy. r-ATG was not
consistently available and its availability was the factor determining
whether the patient received e-ATG or r-ATG.
The overall response to therapy was seen in 29/33
(87.9%) patients. Eight (24.2%) patients achieved complete response, 21
(63.6%) patients had partial response and 4 (12.1%) patients did not
respond (Table II). The median time to achieve complete
response was 9 months.
TABLE II Outcome of Children Treated with Immunosuppressive Therapy
|
Evaluation |
Complete response |
Partial response |
No response |
Alive |
Cumulative Mortality |
|
6 months |
3 |
26 |
2 |
31 |
2 |
|
12 months |
7 |
21* |
2* |
30 |
3 |
|
18 months |
8 |
21# |
1^ |
30 |
3 |
|
Last follow-up |
8 |
21 |
1 |
30 |
3 |
|
*One patient with partial response
relapsed;#One patient who relapsed responded to second course of
rabbit ATG; ^One non-responder continued to be non-response even
after second course of equine ATG.
|
Overall survival: Median follow-up in our study
was 24 (6-102) months. OS at 24 months was 90% (30 patients). One
patient who was a non-responder was alive at 24 months and was receiving
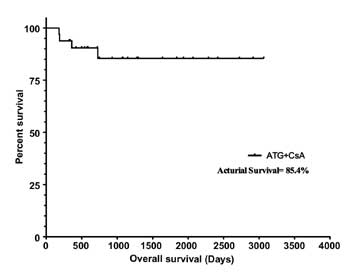
supportive care. The actual survival at 5 years was 85.4% (Fig.1a).
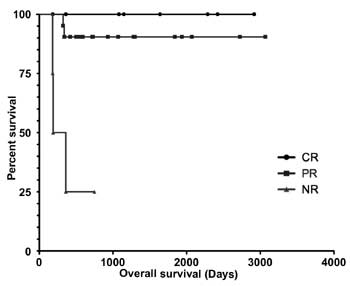
Figure 1b highlights the survival comparison
between the CR, PR and NR group of patients. Three patients died; two at
6 months and one within 12 months of receiving IST. Sepsis, acute
pancreatitis, and pulmonary aspergillosis accounted for one fatality
each. All the 3 patients who died were non-responders to IST.
 |
 |
|
Fig.1 (a) Kaplan-Meier estimates
of overall survival of 33 children and adolescents with aplastic
anemia treated with antithymocyte globulin and ciclosporine; (b)
The comparison of survival among the complete responders,
partial responders and non-responders. Log rank (Mantle-Cox
Test) to compare survival between CR and PR failed to show any
statistical significance (Hazard Ratio=0.26, 95% confidence
interval-0.003 to 24.36, P=0.56.
|
Complications: During the first 90 days after ATG
administration, there were 39 episodes of infection noted in 19
patients. One patient with partial response relapsed 360 days after ATG
administration. However, no clonal disorders were detected in any
patient during the follow-up.
G-CSF: Seventeen patients (51.5%) received G-CSF
for a median duration of 32 days (23-64 days) In total, 94.5% (16/17)
patients who received G-CSF and 81.3% (13/16) patients who did not
receive G-CSF responded to immune suppressive therapy (Relative
Risk=1.16, 95% CI=0.89-1.51, P=0.26). In patients who received G-CSF,
one patient died (5.9%), compared to two deaths (12.5%) in those who did
not receive G-CSF (RR=1.08, 95%CI=0.86-1.34, P=0.51).
The patients who received G-CSF had fewer infectious
complications (7/17 [41.2%] patients; 9/39 [23.1%] infection episodes)
as compared to those who did not receive any G-CSF therapy (12/16 [75%]
patients; 30/39 [76.9%] episodes (RR=0.33, 95% CI=0.16 to 0.70; P=0.002).
The number of days of hospitalization among patients
who received G-CSF versus those who did not receive G-CSF could
not be determined as this data was not available.
Factors predicting outcome: In a stepwise
multivariate regression analysis, none of the factors were predictive of
response or outcome. We compared the covariates between 29 patients
(responders) and 4 patients (non-responders).
The variables that might influence response to IST
and OS, including age, sex, duration of disease before onset of therapy,
clinical presentation, blood counts at presentation, and use of G-CSF
therapy, were tested in a univariate analysis. The following factors had
a significant influence on OS (P<0.1): age<12years, absolute
neutrophil count (ANC) <300/µL, absolute lymphocyte count (ALC)
>1000/µL. The use of G-CSF and other variables did not influence
outcome. Including all these variables in a multivariate Cox regression
analysis, age<12 years ANC<300/µL and ALC>1000 were not
predictive of response/ outcome.
Discussion
HSCT often is the initial treatment in children who
have an HLA-matched sibling donor [1]. The primary treatment of these
children when a matched related donor is unavailable is immune
suppressive therapy [13]. Our study shows an overall response of 87.9%
to immune suppressive therapy with ATG and CsA. The results from other
studies also demonstrate response rates from 74% to 81%, with OS varying
from 67.5% to 88% over 3 to 5 years. Our results confirm an excellent
response to IST among children with aplastic anemia. Unlike most of the
published series, in our study only one patient (3%) relapsed, possibly
due to prolonged duration of CsA therapy and slow tapering.
Our results are better than most of the published
Indian series. In a recently published trial where the response to IST
was compared to HSCT, authors reported only 43.5% response to IST and an
overall survival of 70% post HSCT [14]. Similarly, an earlier series
demonstrated 40% response to IST at 6 months and 45% response to IST at
one year [15]. Our results are definitely superior to previously
published Indian data and are comparable to those from the developed
countries. This is probably due to similar care, both the primary
treatment and the supportive care, being delivered to all children
irrespective of the socio-economic background as the complete expense of
treatment was borne by the armed forces.
The use of G-CSF in the immediate neutropenic phase
after ATG administration is controversial. We demonstrate a reduction in
the number of infectious episodes in the first 90 days after ATG
administration in patients who received G-CSF. Though G-CSF did not
confer any advantage in terms of OS or improved response rates, the
reduction of infectious episodes can translate into shorter
hospitalization and lesser morbidity. These results corroborate those of
Tichelli, et al. [16], where they failed to demonstrate impact of
G-CSF on OS, event free survival or on remission.
None of our patients developed clonal disorders with
use of G-CSF. The results from other researchers have been conflicting
with some studies suggesting a higher risk of clonal disorders with the
use of G-CSF [17,18], while others, including a meta-analysis, failing
to substantiate it [8,19-21]. However, the follow-up time in our study
is too short for a definitive statement and we cannot draw any inference
in this respect from our study.
Although some methods to predict response to IST have
been suggested, but none has been standardized. In an analysis of 300
patients of all ages, younger age, higher pretreatment absolute
reticulocyte count (ARC>25000/µL), and higher pretreatment
absolute lymphocyte count (ALC>1000/µL) were predictive of a
favorable response to IST [22]. In the same study, on subset analysis
authors found that only ARC (and not ALC) correlated with response in
pediatric age group (<18y) [6]. Similarly, in a large European study, a
low pretreatment absolute neutrophil count (ANC<200/µL) was found
to be predictive of response to IST in children [10]. In multi-variate
regression analysis, we failed to demonstrate any predictors of
response/survival. However, due to a small sample size and only four
non-responders, the analysis for predicting variables for survival/
response may be skewed and not definitive.
The limitations of our study include a median
follow-up period of 24 month and a small sample size. Aplastic anemia is
a rare disorder and hence, a single center accrual is scarce. To
summarize, we demonstrate that the outcome with equivalent ATG/CsA as
first line therapy in children is excellent and this corroborates with a
similar response demonstrated from other countries.
Acknowledgements: Commandant, Army Hospital
(Research and Referral Centre) for providing treatment cost. We are also
grateful to the office of DGMS (Army) and the office of DGAFMS for
supporting our endeavor.
Contributors: VN was the principal
investigator and will act as guarantor of the study. VN, AS, SD, and SS
recruited the patients. VS participated in the statistical analysis. VN,
AS, and SD coordinated the research. VN and VS wrote the paper. The
final manuscript was approved by all authors.
Funding: Nil; Competing interests: None
stated.
|
What is Already Known?
• Immunosuppressive therapy using anti-thymocyte
globulin and cyclosporine is effective as first line therapy in
children with aplastic anemia.
What This Study Adds?
• With Immunosuppressive therapy using e-ATG
and CsA, response rates and overall survival of >85% can be
achieved.
• G-CSF may reduce the episodes of infection in these
children but fails to offer any survival advantage or influence
outcome.
|
References
1. Guinan EC. Acquired aplastic anemia in childhood.
Hematol Oncol Clin North Am. 2009 23:171-91.
2. Frickhofen N, Rosenfeld SJ. Immunosuppressive
treatment of aplastic anemia with antithymocyte globulin and
cyclosporine. Semin Hematol. 2000;37:56-68.
3. Frickhofen N, Heimpel H, Kaltwasser JP,
Schrezenmeier H. Antithymocyte globulin with or without cyclosporin A:
11-year follow-up of a randomized trial comparing treatments of aplastic
anemia. Blood. 2003;101:1236-42.
4. Locasciulli A, Oneto R, Bacigalupo A, Socie G,
Korthof E, Bekassy A, et al. Outcome of patients with acquired
aplastic anemia given first line bone marrow transplantation or
immunosuppressive treatment in the last decade: a report from the
European Group for Blood and Marrow Transplantation (EBMT).
Haematologica. 2007;92:11-8.
5. Pongtanakul B, Das PK, Charpentier K, Dror Y.
Outcome of children with aplastic anemia treated with immunosuppressive
therapy. Pediatr Blood Cancer. 2008;50:52-7.
6. Scheinberg P, Wu CO, Nunez O, Young NS. Long-term
outcome of pediatric patients with severe aplastic anemia treated with
antithymocyte globulin and cyclosporine. J Pediatr. 2008;153:814-9.
7. Fuhrer M, Burdach S, Ebell W, Gadner H, Haas R,
Harbott J, et al. Relapse and clonal disease in children with
aplastic anemia (AA) after immunosuppressive therapy (IST): the SAA 94
experience. German/Austrian Pediatric Aplastic Anemia Working Group.
Klin Padiatr. 1998;210:173-9.
8. Kojima S, Hibi S, Kosaka Y, Yamamoto M, Tsuchida
M, Mugishima H, et al. Immunosuppressive therapy using
antithymocyte globulin, cyclosporine, and danazol with or without human
granulocyte colony-stimulating factor in children with acquired aplastic
anemia. Blood. 2000;96:2049-54.
9. Shao Z, Chu Y, Zhang Y, Chen G, Zheng Y. Treatment
of severe aplastic anemia with an immunosuppressive agent plus
recombinant human granulocyte-macrophage colony-stimulating factor and
erythropoietin. Am J Hematol. 1998;59:185-91.
10. Fuhrer M, Rampf U, Baumann I, Faldum A, Niemeyer
C, Janka-Schaub G, et al. Immunosuppressive therapy for aplastic
anemia in children: a more severe disease predicts better survival.
Blood. 2005;106:2102-4.
11. Camitta BM, Thomas ED, Nathan DG, Santos G,
Gordon-Smith EC, Gale RP, et al. Severe aplastic anemia: a
prospective study of the effect of early marrow transplantation on acute
mortality. Blood. 1976;48:63-70.
12. Marsh JC, Ball SE, Cavenagh J, Darbyshire P,
Dokal I, Gordon-Smith EC, et al. Guidelines for the diagnosis and
management of aplastic anaemia. Br J Haematol. 2009;147:43-70.
13. Kurre P, Johnson FL, Deeg HJ. Diagnosis and
treatment of children with aplastic anemia. Pediatr Blood Cancer.
2005;45:770-80.
14. George B, Mathews V, Viswabandya A, Lakshmi KM,
Srivastava A, Chandy M. Allogeneic hematopoietic stem cell
transplantation is superior to immunosuppressive therapy in Indian
children with aplastic anemia—a single-center analysis of 100 patients.
Pediatr Hematol Oncol. 2010;27:122-31.
15. Chandra J, Naithani R, Ravi R, Singh V, Narayan
S, Sharma S, et al. Antithymocyte globulin and cyclosporin in
children with acquired aplastic anemia. Indian J Pediatr.
2008;75:229-33.
16. Tichelli A, Schrezenmeier H, Socie G, Marsh J,
Bacigalupo A, Duhrsen U, et al. A randomized controlled study in
newly-diagnosed severe aplastic anemia patients receiving antithymocyte
globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA
Working Party of the EBMT. Blood. 2011; 117:4434-41.
17. Socie G, Mary JY, Schrezenmeier H, Marsh J,
Bacigalupo A, Locasciulli A, et al. Granulocyte-stimulating
factor and severe aplastic anemia: a survey by the European Group for
Blood and Marrow Transplantation (EBMT). Blood. 2007;109:2794-6.
18. Ohara A, Kojima S, Hamajima N, Tsuchida M,
Imashuku S, Ohta S, et al. Myelodysplastic syndrome and acute
myelogenous leukemia as a late clonal complication in children with
acquired aplastic anemia. Blood. 1997;90:1009-13.
19. Gluckman E, Rokicka-Milewska R, Hann I,
Nikiforakis E, Tavakoli F, Cohen-Scali S, et al. Results and
follow-up of a phase III randomized study of recombinant
human-granulocyte stimulating factor as support for immunosuppressive
therapy in patients with severe aplastic anaemia. Br J Haematol.
2002;119:1075-82.
20. Gordon-Smith EC, Yandle A, Milne A, Speck B,
Marmont A, Willemze R, et al. Randomised placebo controlled study
of RH-GM-CSF following ALG in the treatment of aplastic anaemia. Bone
Marrow Transplant. 1991;7:78-80.
21. Gurion R, Gafter-Gvili A, Paul M, Vidal L, Ben-Bassat
I, Yeshurun M, et al. Hematopoietic growth factors in aplastic
anemia patients treated with immunosuppressive therapy-systematic review
and meta-analysis. Haematologica. 2009;94:712-9.
22. Scheinberg P, Wu CO, Nunez O, Young NS. Predicting
response to immunosuppressive therapy and survival in severe aplastic
anaemia. Br J Haematol. 2009;144: 206-16.
|
|
|
 |
|

