|
|
|
Indian Pediatr 2021;58: 224-228 |
 |
Three vs Four Dose Schedule of Double
Strength Recombinant Hepatitis-B Vaccine in HIV-infected
Children: A Randomized Controlled Trial
|
Prachi Jain, 1
Pooja Dewan,1
Sunil Gomber,1
Bineeta Kashyap2
and Alpana Raizada3
From Departments of 1Pediatrics, 2Microbiology and
3Medicine, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India.
Correspondence to: Dr Pooja Dewan, Professor,
Department of Pediatrics, University College of Medical
Sciences and Guru Teg Bahadur Hospital, Delhi 110 095,
India.
Email:
[email protected]
Received: February 27, 2020; Initial review: April 29,
2020; Accepted: September 17, 2020.
Trial Registration: CTRI/2017/12/010816
|
Objectives: To compare seroprotection rates and the
anti-HBs titers following primary immunization with double
strength (20 µg) recombinant hepatitis B virus (rHBV)
vaccine administered intramuscularly (IM) in a 3-dose (0, 1
and 6 months) vs 4-dose (0, 1, 2 and 6 months) schedule in
HIV-infected children receiving antiretroviral therapy
(ART). An accelerated 3-dose schedule (0, 1, 2 months)
within the 4-dose group was also compared.
Design: Randomized controlled trial.
Setting: Pediatric ART clinic of a
tertiary hospital in Delhi from November, 2017 to April,
2019.
Participants: Fifty (25 per group)
HIV-infected children aged 18 months - 12 years receiving
ART for at least 6 months who had not received any prior
dose of HBV vaccine, and were anti-HBs negative.
Intervention: Group 1 received 20 µg
of rHBV vaccine IM (in deltoid muscle) at 0, 1, and 6
months, and group 2 received 20 µg the same vaccine at 0, 1,
2 and 6 months.
Outcome variables: Anti-HBs titers
and proportion of responders in 3-dose vs 4-dose group at
seventh and twelfth month and at third month after an
accelerated 3-dose schedule.
Result: Median (IQR) anti-HBs titers
at the seventh month were significantly higher in group 2
[225.7 (151-300) IU/L] compared to group 1 [138.2 (35.2-250)
IU/L], but were comparable at the 12th month. Seroprotection
rates were comparable between group 2 and group 1 at 7th
month (96% vs 80%; P=0.19) and 12th month (96% vs
88%; P=0.61). The proportion of good responders were
also comparable between the groups at 7th month and 12th
month (both P=0.29). Accelerated 3-dose schedule
achieved comparable anti-HBs titers [179.9 (130.6-250) IU/L]
and seroprotection rate (92%) one month after completion of
schedule to the standard 3-dose schedule.
Conclusion: A 3-dose double strength
recombinant HBV vaccine schedule offers comparable
seroprotection to 4-dose schedule for HIV-infected children
receiving ART.
Keywords: Accelerated schedule, anti-HBs titer,
Seroprotection.
|
H uman
immunodeficiency virus (HIV) and hepatitis B virus (HBV)
have a high prevalence of co-infection as they share similar
risk factors. HIV infection is also associated with a
greater chance of chronic HBV carrier state, a higher level
of viral replication, increasing its potential for
transmission [1]. HIV and HBV co-infection can accelerate
chronic hepatitis and liver cancer [1] contributing to
morbidity and mortality in HIV-infected individuals.
Immunization is one of the most important public health
measures to prevent HBV infection. How-ever, a suboptimal
seroconversion between 18 to 72% is reported with HBV
vaccine in HIV-infected adults and children [2-4].
In order to improve seroconversion rates
following immunization, several strategies like the use of
double doses [5-9], additional doses [8-11], combination
vac-cines [12], intradermal route for vaccination [8-10],
and adjuvants [13] have been suggested.
The Infectious Diseases Society of
America recommends a 3-dose schedule of double dose (20 µg)
HBV vaccine in children infected with HIV [14]. National
Institutes of Health [15] and Advisory Committee on
Immunization Practices, under the purview of the Centre for
Disease Control, USA [16] recommends a 3-dose schedule of
standard dose (10 µg) recombinant HBV vaccine for
HIV-infected children. The Indian Academy of Pediatrics
recommends double dose of recombinant HBV vaccine in a four
dose schedule at 0, 1, 2 and 6 months in symptomatic
HIV-infected children, and a three-dose schedule in
asymptomatic HIV-infected children at 0, 1 and 6 months
[17]. There is no clear consensus yet regarding the most
appropriate schedule of vaccination for primary HBV
vaccination in HIV-infected children [18]. Highly active ART
(HAART) may foster better immune reconstitution in
HIV-infected children, suggesting that three doses may
suffice to attain adequate seroprotection.
This study was conducted to compare
seroprotection rates, anti-HBs titers and proportion of good
responders following primary immunization with double
strength (20 µg) recombinant HBV vaccine administered in a
3-dose schedule (0, 1 and 6 months) vs 4-dose
schedule (0, 1, 2 and 6 months) in HIV-infected children who
were receiving anti-retroviral therapy (ART) for at least 6
months. We also compared seroprotection rates between the
two 3-dose schedules (0, 1 and 2 month vs 0, 1 and 6
months).
Methods
The study was conducted in Pediatric ART
Clinic of a tertiary hospital in Delhi between November,
2017 and April, 2019. Approval was obtained from the
institutional ethics committee and the trial was registered
with the Clinical Trials Registry of India. Permission was
also obtained from Delhi State AIDS Control Society.
HIV-infected children aged 18 months to
12 years who had been receiving ART for at least 6 months,
were previously unimmunized for hepatitis B and were
seronegative for Hepatitis B virus (HBs antigen negative)
were included. The immunization status was ascertained on
the basis of previous immunization records and a negative
anti-HBs status. Any child with immunological failure, as
defined by National AIDS Control Organization (NACO), was
excluded [19].
A written informed consent was taken from
the parent or guardian. Participants were randomized by
computer generated software using block randomization
technique with variable block sizes. Allocation to 3-dose
and 4-dose groups of the study was done using concealed
envelope technique. Recombinant HBV (rHBV) vaccine
(Biological E. Limited) was administered to the participants
in the immunization clinic while observing all universal
precautions. Children in group 1 received 20 µg of rHBV
vaccine IM (in deltoid muscle) at 0, 1, and 6 months, and
those in group 2 received 20 µg of rHBV vaccine IM at 0, 1,
2 and 6 months. Any adverse event following immunization was
reported to the appropriate authorities.
Two mL venous samples were drawn for
estimation of anti-HBs titers at beginning of the seventh
and twelfth month after receiving the first dose.
Additionally, in the group receiving 4-dose vaccination, a
sample was also drawn one month after the third dose to be
assessed as an accelerated 3-dose schedule (0,1 and 2
months). The sera were separated and stored at -20 ºC.
Anti-HBs titers were estimated after
thawing the stored sera using ELISA-based kits Diapro
Diagnostic Bioprobes Srl). Seroprotection was defined using
an antibody to hepatitis B surface antigen (anti-HBs)
threshold of ³10
IU/L at series completion [20]. Responders and good
responders were defined as participants who had anti-HBs
titers ³10
IU/L and ³100
IU/L at series completion, respectively [20].
The primary outcome variables were
anti-HBs titers and proportion of responders in both groups
after one month (seventh month) of completion of primary
immunization schedule. Secondary outcome variables were
anti-HBs titers and proportion of responders and good
responders at twelfth month in both groups, and proportion
of responders in the accelerated 3-dose schedule.
Sample size was calculated based on the
study by Potsch, et al. [5], where the seroconversion rates
after 4-dose and 3-dose HBV vaccine were 91% and 83%,
respectively. At a non-inferiority margin of 8% with
one-sided type I error rate of 5% and power 80% and assuming
the true difference between seroconversion rates of the two
groups as zero, a sample size of 190 children per group was
calculated. However, with the universal immunization
practices, we did not expect a large cohort of unimmunized
children so we committed to recruit at least 25 children per
group in this study.
Statistical analyses: We used SPSS
software version 22 for analyses. Mann Whitney U test was
used to compare anti-HBs titers between two groups at
different time points. Proportions of non-responders and
good responders were compared by Fisher exact test. Odds
ratio with 95% confidence interval was estimated. P-value
<0.05 was considered significant.
RESULTS
Fifty participants were recruited in the
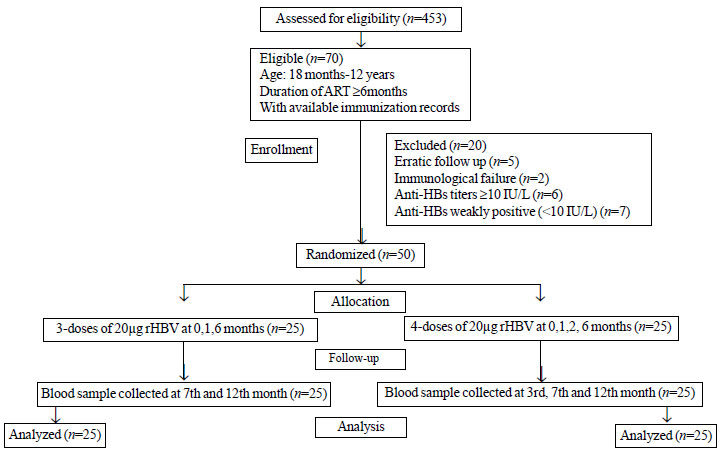
study between November, 2017 and April, 2018. Fig.
1 depicts the flow of participants in the study. The
baseline characteristics of participants in 3-dose and
4-dose groups were comparable (Table I).
 |
|
Fig. 1 Flowchart showing the
recruitment of participants in the study.
|
Table I Baseline Characteristics of Participants in the Study
| Variables |
Three-dose group |
Four-dose group |
|
(n=25) |
(n=25) |
| Age (y) |
7 (4-10) |
11 (9-12) |
| Male sexa |
18 (72) |
15 (60) |
| Weight for age z-score
|
-0.64 (-1.35 to 0.18) |
-1.80 (-2.35 to-1.05) |
| Height for age z-score
|
-0.8 (-2.05 to 0.75) |
-2.50 (-4.3 to-1.6) |
| BMI z-score |
0.06 (-0.92 to 0.75) |
-0.20 (-0.8 to 0.95) |
| On 1st line ARTa |
24 (96) |
23 (92) |
| ART ³24 moa |
14 (56) |
18 (72) |
| CD4 count |
|
|
| Start of ART |
623 (326-959) |
542 (362-893) |
| At enrolment |
1046 (742-1434) |
882 (644-1255) |
| CD4% at enrolment |
34.7 (26.5-37.04) |
31.5 (25.9-37.6) |
|
Three-dose group: Recombinant HBV at 0, 1, 6
month; Four dose group: Recombinant HBV at
0, 1, 2, 6 month; Data expressed as median
(IQR) except ano. (%). ART: anti-retroviral
therapy; BMI: body mass index; P>0.05 for
all variables. |
Table II Anti-HBs Titers and Response With Four-Dose and Three-Dose Schedules of Hepatitis B Vaccination
|
3-dose schedule |
4-dose schedule |
P |
|
(n=25) |
(n=25) |
value |
| Anti-HBs titre
(IU/L)a |
| 7th month |
138.2 (35.3-250) |
225.7 (151-300) |
0.02 |
| 12th month |
166.8 (69.7-250) |
200 (127.5-253) |
0.57 |
| Responders |
| 7th month |
20 (80) |
24 (96) |
0.19 |
| 12th month |
22 (88) |
24 (96) |
0.61 |
| Good responders |
| 7th month |
18 (72) |
22 (88) |
0.29 |
| 12th month |
18 (72) |
22 (88) |
0.29 |
|
Data shown in no. (%) except amedian (IQR);
Responders: Anti-HBs titers ³10 IU/L; Good
responders: Anti-HBs titers ³100 IU/L). |
The median anti-HBs titers and
seroprotection rates achieved in the seventh and twelfth
month in both groups are shown in Table II. No
serious adverse event following immunization (AEFI) was
reported in any child in either group.
The proportion of responders one month
after completion in accelerated schedule were 92% which was
statistically comparable to the corresponding figures in the
3-dose schedule (80%; P=0.42) and 4-dose schedule
(96%; P=0.08). The median anti-HBs titers in the
accelerated schedule were 179.9 (130.6-250) IU/L, which were
comparable to the 3-dose schedule (P=0.26), but
significantly lower than the 4-dose group (P=0.03) at
the seventh month.
DISCUSSION
The study concluded significantly higher
median anti-HBs titers in the 4-dose group as compared to
3-dose group at seventh month, but not at twelfth month. The
proportion of responders and good responders in both groups
were comparable at both time points. This emphasizes that
offering a fourth dose of recombinant hepatitis B vaccine in
HIV-positive children on ART may be unnecessary.
We found seroprotection rates of 96% and
80% one month after HBV vaccination in the 4-dose and 3-dose
schedule, respectively, similar to an adult study [9].
Seroconversion rate of 94% was likewise seen after a 4-dose
(double dose) schedule in unimmunized HIV-infected Indian
children [21]. Similarly, a higher serocon-version rate of
95.4% was seen in seventh, than 88.6% in the twelfth month
after double dose 4-dose vaccination in adults [22].
Seroconversion rate after 3-dose double dose vaccination in
HIV infected children and adults vary from 60-74%
[12,23,24]. The seroconversion rates at both time points in
our study were either comparable or higher than the
aforementioned studies. This may be accounted for by regular
ART intake for atleast six months in the present study
group. Universal ART in children leading to immune
reconstitution and improved vaccine response appears to be a
possible explanation for the robust seroconversion rates of
participants in our study.
The results of our study indicate that a
3-dose schedule may be adequate for primary immunization of
these children with the added advantage of having better
compliance and better use of resources, while ensuring
effective protection against hepatitis B. Antenatal care and
prevention of parent to child transmission (PPTCT) services
have improved nationwide, which have led to more timely
diagnosis in mothers and children. Further, all HIV-infected
children are now routinely receiving ART irrespective of
clinical and immunological staging. Well-equipped ART
clinics with trained doctors and paramedical staff help in
better follow-up, medical care and awareness among these
patients. This has led to the improved immunological status
of HIV positive children and subsequently more effective
response to immunization.
The 4-dose schedule had higher median
value of anti-HBs titers one month after completion of the
vaccination schedule, than the 3-dose schedule. This
difference was not sustained as the titers continued to rise
in the 3-dose group. However, whether the greater proportion
of good responders in the 4-dose group compared to the
3-dose group will offer longer duration of seroprotection,
needs to be confirmed with a longer follow up.
The seroconversion rate increased between
seventh to twelfth month in the 3-dose group but remained
static in the 4-dose group in the present study, similar to
that reported before [25]. The increase in the proportion of
seroconverters over time in our study, however, implied that
those who do not seroconvert initially may show gradual
increment in titers over time. This may be explained by
gradual immune reconstitution with continued ART in
HIV-infected children leading to a delayed immune response
to vaccination.
The timely immunization and sampling were
ensured in the present study without any lost to follow-up.
Earlier studies in children and adults were conducted when
ART was not being administered universally, unlike the
present study where all participants universally received
HAART. Limitation of our study is the small sample size.
Implementation of universal national immunization schedule
and practices makes it difficult to establish a big cohort
of unimmunized HIV positive children. However, the fact that
nearly 11% of children were found to be unimmunized,
emphasizes the need to strengthen the immunization services
for this vulnerable group. Further, due to the
non-availability of viral load and tests for B-cell and
T-cell functions, the non-response to vaccination could not
be explained in a few participants who did not qualify for
severe immunosuppression based on their CD4 counts.
We suggest that three doses of double
strength hepatitis B vaccine may suffice in HIV-infected
children receiving ART in the absence of immunological
failure. The accelerated 3-dose schedule (0, 1, 2 months)
may also be studied for its long term immunogenicity before
it can be considered as an alternative regimen for
vaccination of these children.
Ethics clearance: Institutional
Ethics Committee, UCMS; No. IECHR/2017/32/100 dated 17
October, 2017.
Contributors: PD, PJ, SG, BK, AR:
conceptualized the study; PJ, PD: data collection; BK:
laboratory support; PD, PJ: drafted the manuscript; SG, BK,
AR: critical input. All authors approved the final
manuscript and are accountable for the manuscript.
Funding: Intramural grant,
University College of Medical Sciences.
Competing interests: None stated.
|
What is Already Known?
•
No consensus regarding the optimal number of
doses of hepatitis B vaccine for primary
immunization in HIV-infected children.
What this Study Adds?
•
Three-dose
vaccination offers comparable seroprotection to
four-dose vaccination schedule for hepatitis B
vaccination in unimmunized HIV-infected children
receiving ART.
|
References
1. Soriano V, Puoti M, Bonacini M, et al.
Care of patients with chronic hepatitis B and HIV-coinfection:
Recommendations from an HIV-HBV International Panel. AIDS.
2005;19:221-40.
2. Laurence JC. Hepatitis A and B
immunizations of individuals infected with human
immunodeficiency virus. Am J Med. 2005;118:75S-83S.
3. Van den Berg R, van Hoogstraten I, van
Agtmael M. Non-responsiveness to hepatitis B vaccination in
HIV seropositive patients; possible causes and solutions.
AIDS Rev. 2009;11:157-64.
4. Zuin G, Principi N, Tornaghi R, et al.
Impaired response to hepatitis B vaccine in HIV infected
children. Vaccine.1992; 10:857-60.
5. Potsch DV, Camacho LAB, Tuboi S, et
al. Vaccination against hepatitis B with 4-double doses
increases response rates and antibodies titers in
HIV-infected adults. Vaccine. 2012;30:5973-7.
6. Ni JD, Xiong YZ, Wang XJ, Xiu LC. Does
increased hepatitis B vaccination dose lead to a better
immune response in HIV-infected patients than standard dose
vaccination: A meta-analysis. Int J STD AIDS. 2013;24:
117-22.
7. Scolfaro C, Fiammengo P, Balbo L,
Madon E, Tovo PA. Hepatitis B vaccination in HIV-1-infected
children: Double efficacy doubling the paediatric dose.
AIDS.1996;10:1169-70.
8. Launay O, van der Vliet D, Rosenberg
AR, et al. Safety and immunogenicity of 4 intramuscular
double doses and 4 intradermal low doses vs standard
hepatitis B vaccine regimen in adults with HIV-1: A
randomized controlled trial. JAMA. 2011;305:1-9.
9. Potsch DV, Oliveira MLA, Ginuíno C, et
al. High rates of serological response to a modified
hepatitis B vaccination schedule in HIV-infected adults
subjects. Vaccine. 2010;28:1447-50.
10. Cruciani M, Mengoli C, Serpelloni G,
et al. Serologic response to hepatitis B vaccine with high
dose and increasing number of injections in HIV infected
adult patients. Vaccine. 2009;27:17-22.
11. Rey D, Krantz V, Partisani M, et al.
Increasing the number of hepatitis B vaccine injections
augments anti-HBs response rate in HIV-infected patients.
Effects on HIV-1 viral load. Vaccine. 2000;18:1161-5.
12. Flynn PM, Cunningham CK, Rudy B, et
al. Hepatitis B vaccination in HIV-infected youth: A
randomized trial of three regimens. J Acquir Immune Defic
Syndr. 2011; 54:325-32.
13. Cruciani M, Mengoli C, Serpelloni G,
Mazzi R, Bosco O, Malena M. Granulocyte macrophage
colony-stimulating factor as an adjuvant for hepatitis B
vaccination: a meta-analysis. Vaccine. 2007;25:709-18.
14. Aberg JA, Gallant JE, Ghanem KG,
Emmanuel P, Zingman BS, Horberg MA; Infectious Diseases
Society of America. Primary Care Guidelines for the
Management of Persons Infected With HIV: 2013 Update by the
HIV Medicine Association of the Infectious Diseases Society
of America. Clin Infect Dis. 2014;58:1-10.
15. AIDSinfo. Guidelines for Prevention
and Treatment of Opportunistic Infections in HIV-infected
Adults and Adolescents. April 16, 2015. Accessed August 1,
2020. Available from:
https://aidsinfo.nih.gov/guidelines/html/4/adultand-adolescent-oiprevention-and-treatment-guidelines/344/hbv
16. Mast EE, Margolis HS, Fiore AE, et
al; Advisory Committee on Immunization Practices (ACIP). A
Comprehensive Immunization Strategy to Eliminate
Transmission of Hepatitis B Virus Infection in the United
States: Recommendations of the Advisory Committee on
Immunization Practices (ACIP) Part 1: Immunization of
Infants, Children, and Adolescents. MMWR Recomm Rep.
2005;54:1-31.
17. Shastri DD. Immunization in special
circumstances. In: Balasubramanian S, Shastri DD,
Shah AK, et al, editors. IAP Guide Book on Immunization
2018-2019: By Advisory Committee on Vaccines and
Immunization Practices (ACVIP). 3rd ed: Jaypee Brothers
Medical Publishers; 2020.p406.
18. Hepatitis B vaccines: WHO position
paper – July 2017. Wkly Epidemiol Rec. 2017;92:369-92.
19. National AIDS Control Organization.
Pediatric Anti-Retroviral Therapy Guidelines, 2013.
Available from: http://naco.gov.in/sites/default/files/Pediatric_
14-03-2014.pdf. Accessed April 28, 2020.
20. Shokrgozar MA, Shokri F. Enumeration
of hepatitis B surface antigen-specific B lymphocytes in
responder and non-responder normal individuals vaccinated
with recombinant hepatitis B surface antigen. Immunology.
2001;104:75-9.
21. Bose D, Chandra J, Dutta R, et al.
Immune response to double dose hepatitis-B vaccine using
four dose schedule in HIV infected children. Indian J
Pediatr. 2016;83:772-6.
22. Chaiklang K, Wipasa J, Chaiwarith R,
Praparattanapan J, Supparatpinyo K. Comparison of
immunogenicity and safety of four doses and four double
doses vs. standard doses of hepatitis B vaccination in
HIV-infected adults: A randomized, controlled trial. PLoS
One. 2013;8:e80409.
23. Siddiqui SA, Maurya M, Singh DK,
Srivastava A, Rai R. Double dose versus standard dose
hepatitis B vaccine in HIV-infected children: A randomized
controlled trial. Indian Pediatr. 2017;54:1017-20.
24. Cornejo-Suarez P, Volkow-Fernandez P,
Escobedo-Lopez K, Vilar-Compte D, Ruiz-Palacios G, Soto-
Ramirez LE. Randomized controlled trial of hepatitis B virus
vaccine in HIV-1 infected patients comparing two different
doses. AIDS Res Ther. 2006;3:9.
25. Launay O, Rosenberg AR, Rey D, et al.
Long-term immune response to hepatitis B virus vaccination
regimens in adults with human immunodeficiency virus 1:
Secondary analysis of a randomized clinical trial. JAMA Int
Med. 2016;176:603-10.
|
|
|
 |
|

