|
|
|
Indian Pediatr 2020;57: 222-227 |
 |
Comparison of Phenytoin, Valproate and
Levetiracetam in Pediatric Convulsive Status Epilepticus: A
Randomized Double-blind Controlled Clinical Trial
|
Vinayagamoorthy Vignesh, Ramachandran Rameshkumar
and Subramanian Mahadevan
From Division
of Pediatric Critical Care, Department of Pediatrics,
Jawaharlal Institute of Postgraduate Medical Education and
Research, Puducherry, India.
Correspondence to: Dr
Rameshkumar Ramachandran, Associate Professor, Division of
Pediatric Critical Care, Department of Pediatrics,
Jawaharlal Institute of Postgraduate Medical Education and
Research (JIPMER), Puducherry, India.
Email:
[email protected]
Received: June
26, 2019;
Initial review: November 04, 2019;
Accepted: January 20, 2020.
Trial Registration: CTRI/2016/05/006908.
|
|
Objective: To compare
the efficacy of phenytoin, valproate, and levetiracetam in
the management of pediatric convulsive status epilepticus.
Design: Randomized double-blind
controlled clinical trial.
Setting:
Pediatric critical care division in a tertiary care
institute from June, 2016 to December, 2018.
Participants: 110 children aged three month
to 12 year with convulsive status epilepticus.
Intervention: Patients not responding to
0.1 mg/kg intravenous lorazepam were randomly assigned
(1:1:1) to receive 20 mg/kg of phenytoin (n=35) or valproate
(n=35) or levetiracetam (n=32) over 20 minutes. Patients
with nonconvulsive status epilepticus, recent hemorrhage,
platelet count less than 50,000 or International normalized
ratio (INR) more than 2, head injury or neurosurgery in the
past one-month, liver or kidney disease, suspected or known
neurometabolic or mitochondrial disorders or structural
malformations, and allergy to study drugs; and those who
were already on any one of the study drugs for more than one
month or had received one of the study drugs for current
episode, were excluded.
Outcome measure:
The primary outcome was the proportion of patients that
achieved control of convulsive status epilepticus at the end
of 15 minutes after completion of the study drug infusion.
Secondary outcomes were time to control of seizure, rate of
adverse events, and the requirement of additional drugs to
control seizure, length of ventilation, hospital stay, and
functional status after three months (Glasgow Outcome
Scale).
Results: The study was
stopped after the planned mid-interim analysis for futility.
Intention to treat analysis was done. There was no
difference in primary outcome in phenytoin (31/35, 89%),
valproate (29/35, 83%), and levetiracetam (30/32, 94%)
(P=0.38) groups. There were no differences between the
groups for secondary outcomes. One patient in the phenytoin
group had a fluid-responsive shock, and one patient in the
valproate group died due to encephalopathy and refractory
shock.
Conclusions: Phenytoin,
valproate, and levetiracetam were equally effective in
controlling pediatric convulsive status epilepticus.
Keywords: Anti-epileptic drugs, Management,
Outcome Seizure.
|
|
Convulsive status epilepticus (CSE) is the most
common time-bound pediatric neurological emergency worldwide, where
delayed control is associated with neurological sequelae and risk of
mortality [1]. Half of the children in an Indian emergency department
had convulsive status epilepticus at their first presentation without
having any history of prior seizure [2]. The available evidence supports
that benzodiazepines should be the drugs of first choice for CSE [3].
Subsequently, intravenous phenytoin/fosphenytoin remains the most used
antiepileptic drug. The other reasonable options are valproate,
levetiracetam and phenobarbital. There is insufficient evidence to
support the use of one particular drug over the others [1,4,5]. Thus, we
compared the efficacy of phenytoin, valproate, and levetiracetam in
pediatric convulsive status epilepticus. We hypothesized that
levetiracetam would be associated with better control of seizures as
compared to phenytoin and valproate in pediatric convulsive status
epilepticus.
METHODS
This
randomized, double blinded-controlled clinical trial was
conducted in the Division of pediatric critical care of a
tertiary-care academic institution between June, 2016 to
December, 2018. The institutional ethics committee approved the
study and written informed consent was obtained from
parents/legal guardians. Children aged 3 month to 12 years with
convulsive status epilepticus (clonic, tonic, tonic-clonic, and
myoclonic, focal or generalized) were enrolled. Children with
either of the following conditions were excluded (i)
non-convulsive status epilepticus, (ii) active or recent
hemorrhage (less than one week) from any site, (iii) documented
platelet count less than 50,000, or international normalized
ratio more than two, (iv) head injury or neurosurgery in the
past one month, (v) acute or chronic liver or kidney disease,
(vi) suspected or known neurometabolic or mitochondrial
disorders or structural malformations, (vii) known or suspected
allergy to any of the study drugs, (viii) patient with epilepsy
already on levetiracetam (more than 20 mg per kg per day) or
valproate (more than 20 mg per kg per day) or phenytoin (more
than 5 mg per kg per day) for more than one month, and (ix)
patients who have received the appropriate dose of study drug(s)
for the current episode of convulsive status epilepticus.
Convulsive status epilepticus was defined as continuous seizure
activity or recurrent seizure activity without regaining
consciousness, lasting more than five minutes [6,7]. Status
epilepticus and its etiology were classified as per
International League Against Epilepsy guidelines [6].
A
computer-generated and unstratified block randomization with
variable block sizes of three, six, and nine were used. A person
not involved in the study performed the random number
allocation. Individual assignments were placed in sequentially
numbered opaque sealed envelopes (SNOSE) with a three-component
alphanumerical code. The envelope contained an instruction slip
about the preparation of the study drug. Nursing personnel, who
was not part of the research team, opened the envelope and
prepared the study drug concentration of 5 mg/mL in 0.9% normal
saline dilution in the syringe. Each syringe was labeled with
the same alphanumerical code, and study drug dose (4 mL per kg
over 20 minute). The person who prepared the study drug was
blinded to the patient’s identity. Injection phenytoin sodium
(Ciroton, 2 mL per 100 mg, Ciron Pharmaceuticals, India),
injection sodium valproate (Valprol, 5 mL per 500 mg, Intas
Pharmaceuticals, India) and injection levetiracetam (Levesam, 5
mL per 500 mg, Abbott Ind. Ltd, India) were used in this study.
The Institute’s central pharmacy supplied the study drugs. The
participants, treating doctors and nurses administering the
drugs, as well as the investigators and research personnel, were
unaware of the treatment assignments until control of seizure.
Later, the study drug was unblinded to the treating team to
continue maintenance therapy. The person who collected the data
and entered it into the datasheet, and the study statistician
were unaware of the treatment assignments until final analyses.
At the time of analysis, another person not involved in the
study and SNOSE preparation decoded the treatment assignment by
using the code from the online stored datasheet.
Enrolled patients were managed by stabilizing the airway,
breathing and circulation, and using intravenous lorazepam 0.1
mg/kg in the pediatric emergency room. Patients not responding
to intravenous lorazepam received the study drug at the dose of
20 mg kg over 20 minutes as an intravenous infusion. If
convulsions were not controlled with the study drug or there was
recurrence of seizure after control by study drug, additional
antiepileptic drugs were administered as per the treating team’s
discretion. The patients were shifted to the pediatric intensive
care unit or ward for further management and etiological workup,
as per unit protocol. Survivors were followed for three months
post-discharge. The functional status was assessed using Glasgow
outcome scale score, which ranges from one to five (higher the
score better the neurological function).
The primary
outcome was the proportion of patients who achieved control of
convulsive status epilepticus at the end of 15 minutes after
completion of study drug infusion (i.e., 35 minutes after
starting the study drug infusion). The secondary outcomes were
(i) time (minutes) taken to control seizure from the initiation
of study drug infusion, (ii) proportion of patients who required
additional drug to abort clinical seizures, (iii) rate of
adverse events, (iv) length of mechanical ventilation if
ventilated; (v) hospital stays including pediatric intensive
care stay, (vi) in-hospital mortality, and (vii) functional
status at three months of follow-up by Glasgow Outcome Scale.
Based on a study by Mundlamuri, et al. [8], control of
convulsive status epilepticus by phenytoin and valproate was
found to be at 68%. We, therefore, assumed that levetiracetam
might increase the control rate to 88%. With a two-sided alpha
of 5% and 80% power, 68 patients were needed in each group
(nQuery + nTerim3.0 version software). Interim analysis was
planned at the end of 50% enrollment. The trial progress was
reviewed yearly by the institute’s ethics and data and safety
monitoring committee, including an independent statistician who
was also a physician. The trial had to be stopped prematurely
after the planned interim analysis contended that it was futile
to continue the study further.
Statistical analyses:
Data of all the patients were analyzed according to their
assigned groups (Intention to treat). The normality of data was
checked with the Kolmogorov-Smirnov Z test. Continuous data were
compared by one-way analysis of variance (ANOVA) if normally
distributed or by Kruskal-Wallis test if non-normally
distributed and proportions with Chi-square test. All tests were
two-tailed, and a P value <0.05 was considered statistically
significant. SPSS version 20.0 (IBM SPSS Statistics, Armonk, NY)
and Epi Info 7 (7.0.9.7, CDC, Atlanta, GA) were used for data
analysis.
RESULTS
The study
flow is depicted in Fig.1. The baseline
characteristics and investigations were comparable in the study
groups (Table I). The median duration of
seizure, before enrollment, was 10 minutes in each group. Seven
(7%) of patients received normal saline bolus and six (6%)
patients received vasoactive therapy. Five patients in each
group received osmotherapy for cerebral edema. Antibiotics and
antivirals were given in 40 (39.2%) and 16 (16%) patients,
respectively (Table I). Computerized tomography
was done in 55 (54%) patients, and magnetic resonance imaging
was done in 41 (40%) patients. Abnormalities were found in 18
studies, with tubercular involvement in two children and
multiple neurocysti-cercosis in one child. Control of convulsive
status epilepticus was higher in the levetiracetam group (94%)
as compared to the phenytoin group (89%) and valproate group
(83%), though statistically no difference was found (P=0.38).
The mean time to control of seizure was three minutes (P=0.42).
Additional drug to control the seizure after control of seizure
by study drug was higher in the phenytoin group (26%) as
compared to the valproate (14%) and levetiracetam (13%) groups.
Twenty-eight patients (27.5%) were shifted to the pediatric
intensive care unit; mean stay was significantly lower in the
phenytoin group (Table II). One patient died in
the valproate group due to encephalopathy and refractory shock;
this death was not thought to be due to the study drug. No
intervention-related serious adverse event was noted, except for
one patient in the phenytoin group who had a fluid responsive
shock.
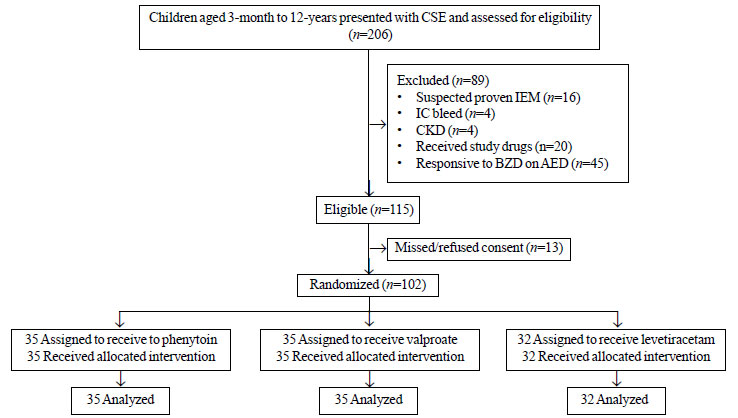 |
| Fig. 1 Study flow.
CSE: Convulsive status epilepticus; IEM: Inborn error of
metabolism; IC bleed: Intracranial bleed; CKD: Chronic
kidney disease; BZD: Benzodiazepine; AED: Anti epileptic
medication. |
TABLE I Baseline Characteristics of Children With Convulsive Status Epilepticus in the Three Treatment Groups
|
Variable
|
Phenytoin group
(n=35) |
Valproate group
(n=35) |
Levetiracetam
group (n=32) |
P value
| |
*Age (mo) |
44 (43) |
59 (44) |
58 (50) |
0.32 | |
Male |
19 (54.3) |
21 (60) |
18 (56.2) |
0.89 | |
*Body Mass Index, z score |
- 1.7 (2) |
- 1.1 (1.9) |
- 1.6 (2) |
0.32 | |
*(cm) Head circumference |
46.4 (4.2) |
48.3 (3.5) |
47 (4.8) |
0.16 | |
#PRISM-III |
5 (3 - 8) |
4 (2-7) |
3 (0-5) |
0.17 | |
#Duration of seizure, prior to enrollment (min) |
10 (10 - 23) |
10 (10-15) |
10 (10-18) |
0.57 | |
Fever history |
23 (66) |
15 (43) |
15 (47) |
0.13 | |
Classification of status epilepticus, n (%) | | | |
0.44 | |
Generalized convulsive |
26 (74) |
31 (88) |
24 (75) | | |
Focal motor |
5 (14) |
2 (6) |
6 (19) | | |
Focal onset evolving into bilateral convulsive SE |
4 (11) |
2 (6) |
2 (6) | | |
Family history of seizure disorder |
4 (11) |
2 (6) |
1 (3) |
0.38 | |
Developmental delay |
5 (14) |
8 (23) |
5 (16) |
0.60 | |
Hypocalcemia |
4 (11) |
3 (9) |
2 (6) |
0.76 | |
Abnormal CT head (n=55) |
4 / 23 (17) |
3/16 (19) |
1/16 (6) |
0.37 | |
‡MRI Brain* (n=41) |
5 / 12 (42) |
2/13 (15) |
5/16 (31) |
0.43 | |
‡Electroencephalographic abnormality |
15 / 27 (56) |
17 /29 (59) |
12/21 (57) |
0.97 | |
Cerebrospinal fluid pleocytosis |
10 (29) |
7 (20) |
4 (13) |
0.27 | |
Etiology | | | |
0.28 | |
Acute |
16 (46) |
7 (20) |
14 (44) | | |
Remote |
9 (25) |
7 (20) |
5 (16) | | |
Acute on remote |
1 (3) |
2 (6) |
- | | |
Febrile status epileptics |
2 (6) |
2 (6) |
2 (6) | | |
Unknown (ie, cryptogenic) |
7 (20) |
17 (48) |
11 (34) | | |
All values in no. (%) except *mean (SD) or #median (IQR); Hypocalcemia defined as ionized calcium less than one mmol/L or total serum calcium less than 8.5 mg/dL; PRISM: *Pediatric risk mortality score; CT: Computer tomography; MRI: Magnetic resonance imaging; ‡done during the follow-up. |
TABLE II Outcome in Children With Convulsive Status Epilepticus in the Three Treatment Groups (N=102)
|
Outcome |
Phenytoin group |
Valproate group |
Levetiracetam |
P value | |
(n=35) |
(n=35) |
group (n=32) | | |
Primary outcome, n (%) |
31 (89) |
29 (83) |
30 (94) |
0.38 | |
Secondary outcomes | | | | | |
Time to control seizure (min), mean (SD) |
3 (1.2) |
3.2 (1.4) |
3.1 (1.3) |
0.42 | |
‡Additional drug to control the seizure, n (%) |
4 (11.4) |
6 (17) |
2 (6) |
0.38 | |
$Additional drug to control seizure, n (%) |
8 / 31 (26) |
4 / 29 (14) |
4 / 30 (13) |
0.35 | |
Mechanical ventilation, n (%) |
7 (20) |
5 (14) |
3 (9) |
0.47 | |
Length of mechanical ventilation (d), mean (SD) |
2 (1.2) |
7 (5.5) |
3 (1.7) |
0.08 | |
PICU shifting, n (%) |
15 (43) |
7 (20) |
6 (19) |
0.04 | |
PICU stay (d), mean(SD) |
4 (2.4) |
10 (4.5) |
6 (3.7) |
0.005 | |
Hospital stay (d), mean (SD) |
6.1 (4.1) |
5.5 (5.4) |
7 (7.4) |
0.55 | |
Functional status (at discharge), n (%) | | | |
0.46 | |
GOS score-1 |
- |
1 (3) |
- | | |
GOS score-3 |
- |
1 (3) |
1 (3) | | |
GOS score-4 |
8 (23) |
12 (34) |
6 (19) | | |
GOS score-5 |
27 (77) |
21 (60) |
25 (78) | | |
#Functional status (at 3 mo), n (%) | | | |
0.06 | |
GOS score-3 |
- |
- |
1 (3) | | |
GOS score-4 |
3 (9) |
10 (29) |
3 (9) | | |
GOS score-5 |
32 (91) |
24 (71) |
28 (88) | | |
Mortality, n (%) |
- |
1 (2.8) |
- |
- | |
Adverse event, n (%) |
1 (2.8)* |
- |
- |
- | |
PICU: Pediatric Intensive Care Unit; GOS: Glasgow Outcome Scale; *fluid responsive shock, #n=34 for valproate group, $after control of seizure by study drug; ‡no response to study drug. |
DISCUSSION
The present randomized controlled study found that phenytoin,
valproate, and levetiracetam are safe and equally efficacious in
the management of pediatric status epilepticus. Our study
findings are consistent with recent controlled studies. A study
in adults [8], compared phenytoin (20 mg per kg), valproate (30
mg per kg) and levetiracetam (40 mg per kg) after 0.1 mg per kg
of lorazepam found that there was no difference in the control
of generalized convulsive status epilepticus (68% vs. 68% vs.
78%) and 6% of levetiracetam group patients had post-ictal
psychosis. A more recent study [9] in both children and adults,
comparing fosphenytoin (20 mg of phenytoin equivalent per kg),
valproate (40 mg per kg) and levetiracetam (60 mg per kg), found
that cessation of status epilepticus and improvement in the
level of consciousness at 60 minutes of starting study drug
infusion was similar in all three groups (45%, 46%, and 47%,
respectively). The ConSEPT study [10] and the EcLiPSE study [1]
compared 20 mg per kg phenytoin and 40 mg per kg levetiracetam.
Clinical cessation of seizure activity in children with status
epilepticus refractory to benzodiazepine was similar in both
studies (60% vs. 50% and 64% vs. 70%, respectively) [1,10].
Isguder, et al. [11] reported that control of status
epilepticus in pediatric patients was 71.8% with valproate and
levetiracetam. The lower rate of seizure control could be due to
a longer median duration of status epilepticus of 75 minutes, as
compared to 10 minutes in our study.
A meta-analysis in
pediatric status epilepticus found that valproate had a higher
efficacy of 75.7% as compared to levetiracetam (68.5%) and
phenytoin (50.2%) after administration of benzodiazepine [12].
Another meta-analysis of five randomized studies, which included
one pediatric study (valproate vs. phenytoin), with insufficient
information about random sequence generation and allocation
concealment, found that there was no difference in clinical
seizure control in both direct (valproate vs. phenytoin; 77% vs.
76% and levetiracetam vs. phenytoin; 72% vs. 68%) and indirect
(levetiracetam vs. valproate; 72% vs. 77%) comparison [13]. Our
study found a relatively higher control rate of seizure; as
compared to other published studies [1,,8-13], possibly due to
shorter duration of seizures before treatment in our study.
We found that the proportion of patients shifted to the
pediatric intensive care unit was significantly higher in the
phenytoin group. This could be due to the underlying illness in
addition to the drug effects on neurological function. Valproate
is reported to have a lower risk of cardiorespiratory compromise
and a lack of sedative effect [14,15].
Our study had
certain methodological differences from other similar studies.
We assessed the absence of seizure 15 minutes after completion
of study drug infusion, i.e. 35 minutes after starting the
infusion, and the mean time taken to control of seizure was
three minutes. We randomized the patients who did not respond to
the benzodiazepine and used intention to treat analysis. This
finding differs from the EcLiPSE study, which found that median
time from randomization to the cessation of convulsive status
epilepticus was similar in phenytoin and levetiracetam group
(45-minute vs. 35-minute) and ConSEPT study assessed the
clinical cessation of seizure activity five minutes after
completion of infusion of the study drug with a different
infusion time used for administration of study drugs (over five
minutes and over 20 minutes) [1,10]. Another controlled study by
Kapur, et al. [9] assessed the absence of seizure and recovery
of consciousness after 60 minutes of starting the study drug
infusion, and emergency unblinding before 60 minutes was
considered a protocol deviation. Hence, the time limit followed
for assessment of primary endpoint in our study is in line with
the International League Against Epilepsy operational time point
(t1 and t2) of status epilepticus [6].
Apart from the
duration of seizure, age and underlying etiologies have a
different impact on the prognosis of neurological outcome, even
if assuming a similar seizure type [6]. In our study, these
prognostic factors were not analyzed. Though it is difficult to
differentiate the role of each of the prognostic factors, data
from larger studies could allow for redefining of the risk of
long-term neuro-morbidity. Another strength of our study was
that the neurological outcome at three-month was assessed. This
is in contrast to six previous open-labeled controlled studies
with valproate and two with levetiracetam, no follow-up details
were provided [5]. Our study did not include the recovery of
postictal consciousness, long term drug-related adverse effects,
and behavioral assessment. Future studies with large sample
size, preferably multicentric, should focus on children with
different etiologies, including liver and hematological
diseases, with stratification of the duration of seizure and
convulsive versus non-convulsive seizures.
In conclusion,
our study shows that phenytoin, valproate, and levetiracetam are
equally effective in controlling seizure in the management of
pediatric convulsive status epilepticus with a similar
neurological outcome at three-month follow-up.
Acknowledgments: S Raja Deepa, JIPMER Campus, Puducherry, India
for review and editing of the manuscript; Mr Rakesh Mohindra,
Punjab University, Chandigarh, India. Mrs Thenmozhi M for
helping with statistical analysis and Mrs. Harpreet Kaur, Punjab
University, Chandigarh, India, Mrs. Neelima Chadha (Tulsi Das
Library, PGIMER, Chandigarh, India) for helping with medical
literature search.
Contributors: VV,RR,SM: Management of
the patients and study supervision. VV: collected the data,
reviewed the literature and drafted the first manuscript: SM:
contributed for protocol development, review of literature and
manuscript. RR: conceptualized the study, reviewed the
literature and critically reviewed the manuscript. All authors
approved the final version of the manuscript. RR: is the
guarantor of the paper.
Funding: In part by the
institutional and departmental fund;
Competing interest:
None stated.
|
WHAT THIS STUDY ADD?
•
Phenytoin, valproate, and levetiracetam at a dose of 20
mg/kg infusion over 20 minute were equally efficacious in the
management of pediatric convulsive status epilepticus not
responding to single dose of lorazepam, and patients had similar
neurological outcome at three-month follow-up.
|
REFERENCES
1. Lyttle MD, Rainford
NEA, Gamble C, Messahel S, Humphreys A, Hickey H, et al.
Levetiracetam versus phenytoin for second-line treatment of
paediatric convulsive status epilepticus (EcLiPSE): A
multicentre, open-label, randomised trial. Lancet.
2019;393:2125-34.
2. Gulati S, Kalra V, Sridhar MR.
Status epilepticus in Indian children in a tertiary care
center. Indian J Pediatr. 2005;72:105-8.
3. McTague
A, Martland T, Appleton R. Drug management for acute
tonic-clonic convulsions including convulsive status
epilepticus in children. Cochrane Database Syst Rev.
2018;1:CD001905.
4. Verrotti A, Ambrosi M, Pavone P,
Striano P. Pediatric status epilepticus: Improved management
with new drug therapies? Expert Opin Pharmacother.
2017;18:789-98.
5. Trinka E, Kälviäinen R. 25 years
of advances in the definition, classification and treatment
of status epilepticus. Seizure. 2017;44:65-73.
6.
Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE,
Shinnar S, et al. A Definition and Classification of Status
Eepilepticus – Report of the ILAE Task Force on
Classification of Status Epilepticus. Epilepsia.
2015;56:1515-23.
7. Brigo F, Sartori S. The new
definition and classification of status epilepticus: What
are the implications for children? Epilepsia.
2016;57:1942-3.
8. Mundlamuri RC, Sinha S,
Subbakrishna DK, Prathyusha PV, Nagappa M, Bindu PS, et al.
Management of generalised convulsive status epilepticus
(SE): A prospective randomised controlled study of combined
treatment with intravenous lorazepam with either phenytoin,
sodium valproate or levetiracetam—Pilot study. Epilepsy Res
2015; 114:52-8.
9. Kapur J, Elm J, Chamberlain JM,
Barsan W, Cloyd J, Lowenstein D, et al. Randomized trial of
three anticonvulsant medications for status epilepticus. N
Engl J Med. 2019;381:2103-13.
10. Dalziel SR, Borland
ML, Furyk J, Bonisch M, Neutze J, Donath S, et al.
Levetiracetam versus phenytoin for second-line treatment of
convulsive status epilepticus in children (ConSEPT): an
open-label, multicentre, randomised controlled trial. Lancet
2019;393:2135-45.
11. Ýþgüder R, Güzel O, Ceylan G,
Yýlmaz Ü, Aðýn H. A comparison of intravenous levetiracetam
and valproate for the treatment of refractory status
epilepticus in children. J Child Neurol. 2016;31:1120-6.
12. Yasiry Z, Shorvon SD. The relative effectiveness of
five antiepileptic drugs in treatment of
benzodiazepine-resistant convulsive status epilepticus: a
meta-analysis of published studies. Seizure. 2014;23:167-74.
13. Brigo F, Bragazzi N, Nardone R, Trinka E. Direct and
indirect comparison meta-analysis of levetiracetam versus
phenytoin or valproate for convulsive status epilepticus.
Epilepsy Behav. 2016;64:110-5.
14. Mehta V, Singhi
P, Singhi S. Intravenous sodium valproate versus diazepam
infusion for the control of refractory status epilepticus in
children: A randomized controlled trial. J Child Neurol.
2007;22:1191-7.
15. Mishra D, Sharma S, Sankhyan N,
Konanki R, Kamate M, Kanhere S, et al. Consensus guidelines
on management of childhood convulsive status epilepticus.
Indian Pediatr. 2014;51:975-90.
16. Dawrant J, Pacaud
D. Pediatric hypocalcemia: making the diagnosis. CMAJ.
2007;177:1494-7.
|
|
|
 |
|

