|
|
|
Indian Pediatr 2020;57: 218-221 |
 |
Efficacy, Tolerability and Serum Phenytoin
Levels after Intravenous Fosphenytoin Loading Dose in Children
with Status Epilepticus
|
|
Kavita Srivastava, Shirish Bhartiya,
Vrushabh Gavli, Rahul Patil and Surekha Rajadhyaksha
From Pediatric Neurology Unit, Department of Pediatrics, Bharati
Vidyapeeth Deemed University Medical College, Pune, India.
Correspondence to: Dr Kavita Srivastava, Professor in Pediatrics, 3rd
floor, Bharati Hospital, Katraj, Pune 411 043, India. Email:
[email protected]
Received: July 05, 2018;
Initial review: December 03, 2018;
Accepted: December 04, 2019.
|
|
Objective: To evaluate
the efficacy and tolerability of intravenous fosphenytoin in
children with status epilepticus, and resulting serum total
phenytoin levels.
Methods: In this
prospective study, 51 children aged less than 18 years
received intravenous loading dose of fosphenytoin (18-20
mg/kg). Serum total phenytoin levels were estimated at 90
-100 minutes. Outcomes studied were (i) seizure control and
local and/or systemic adverse effects in next 24 hours and
(ii) phenytoin levels and its correlation with dose
received, seizure control and adverse effects.
Results: The actual dose of fosphenytoin
received varied from 15.1 to 25 mg/kg. Seizures were
controlled in 45 (88%) children and, two required additional
dose of 10 mg/kg. None of the children showed any local or
systemic adverse effects. Serum total phenytoin levels were
in the therapeutic range (10-20 µg/mL) in 12 (23.5%),
sub-therapeutic in 16 (31.3%) and supra-therapeutic in 25
(49%) children. There was weak correlation of the phenytoin
levels with dose of fosphenytoin received, seizure control,
or adverse effects.
Conclusion:
Intravenous fosphenytoin loading dose of 20 mg/kg is
effective in controlling seizures in 88% of children with
status epilepticus, with a good safety profile. Seizure
control and adverse effects appear to be independent of
serum total phenytoin levels achieved.
Keywords: Anticonvulsant, Management, Seizure
control, Therapeutic levels.
|
|
Intravenous Phenytoin (PHT) is the first long-acting drug (after
benzodiazepines) recommended for the treatment of status epilepticus
[1,2]. Fosphenytoin (FOS) is a pro-drug which is rapidly converted to
PHT and preferred due to less incidence of thrombophlebitis and
cardiotoxicity [3,4]. PHT follows non-linear kinetics, causing
unpredictable blood levels, with higher levels associated with cardiac
arrhythmias and hypotension. PHT concentrations may be influenced by
ethnicity due to its hepatic metabolism through cytochrome P450 enzymes
[5,6].
While it is recommended to maintain serum total PHT levels
in the therapeutic range of 10 to 20 µg/mL, monitoring is not done
routinely in India, possibly due to cost or feasibility issues. Efficacy
and safety of FOS have been stressed by few authors, and others have
evaluated the pharmacokinetics in status epilepticus [7-11]. Hence this
study was done to evaluate the efficacy and tolerability of loading dose
of 20 mg/kg of intravenous FOS in children admitted for status
epilepticus and to, correlate the serum PHT levels after 90-100 minutes
of loading with actual dose received, seizure control and adverse
effects.
METHODS
This was an
observational study conducted in emergency ward and pediatric intensive
care unit (PICU) of a medical college affiliated hospital over 10 months
(December, 2016 to September, 2017). Institutional Ethics Committee
approval was taken, and written informed consent from parents was
obtained to participate in the study.
Children aged one month to
18 years with status epilepticus were enrolled in the study. Status
epilepticus was defined as seizure duration of more than 30 minutes or
two or more seizures without regaining consciousness. Those who were
already on oral PHT or loaded with any other antiepileptic drug outside
the hospital were excluded.
All patients received a standard
protocol for securing airway, oxygenation, and circulation. IV Lorazepam
0.1 mg/kg followed by IV FOS [(Brand Fosolin, Zydus Cadilla), content:
50 mg/mL of phenytoin equivalents (PE)] at a dose of 20 mg/kg for
estimated weight (or actual weight, if known) was administered.
Blood sample (5 mL) was obtained at 90 to 100 minutes (after loading
dose) to determine the serum total PHT levels, serum albumin, and
creatinine. The PHT levels were estimated using CLIA (Chemiluminescence
immunoassay) method by Immulite 1000 machine (Siemens, Los Angeles CA
90045 USA. If there was a breakthrough seizure, a second dose of 10
mg/kg of IV FOS was administered.
A detailed history of
perinatal events, development, family history, and etiology of seizures
were recorded, along with physical and neurological examination. Seizure
control was defined as cessation of any clinical seizure activity. The
total duration of seizure, need of additional dose/ other anti-epileptic
drugs, and any adverse effects were recorded for the next 24 hours. The
patients were followed till discharge/death, and final outcome noted.
Accurate weight was obtained after recovery and the actual dose
received in mg/Kg was recalculated. Optimal dose was defined as 18-20
mg/kg of PE, subnormal if less than 18 mg/kg and supra-normal if more
than 20 mg/kg. PHT levels were considered to be in the therapeutic range
between 10-20 µg/mL, below 10 µg/mL as sub-therapeutic, above 20 µg/mL
as supra-therapeutic and more than 40 µg/mL to be in toxic range. The
outcome studied for efficacy was the number of patients with clinical
control of seizures in next 24 hours. For tolerability, number of
patients with local (cording of vein, erythema, and swelling at IV site)
and systemic adverse effects (e.g. vomiting, nystagmus, ataxia etc.) was
recorded.
Statistical analysis: Linear regression of serum PHT
levels with actual loading dose received was plotted, and Pearson
correlation coefficient was computed. PHT levels were further analyzed
to see whether they correlated with seizure control and adverse effects.
RESULTS
Fifty-one children (54.9% males)
were prospectively enrolled. Age distribution was as follows: below one
year (n=9), 1-5 years (n=19), 5-10 years (n=14) and more than ten years
(n=9). The seizure types were generalized tonic-clonic seizure (n=40),
focal seizure with impaired awareness (n=8), or evolving into bilateral
convulsive seizure (n=3). Among the fifteen children who were already
diagnosed with epilepsy, 11 were on antiepileptic medications: valproate
(n=2), topiramate (n=2), oxcarbazepine (n=2), levetiracetam (n=2) and
nitrazepam (n=2). One child was on both valproate and levetiracetam.
Etiologies were febrile status epilepticus (n=10), prior brain insult
(n=7) meningitis (n=6), traumatic brain injury (n=4), sepsis (n=2),
subdural hematoma (n=1), neurocysticercosis (n=1), metabolic disorder
(n=1) and unknown (n=19).
|Among the 51 children, 32 (62.7%)
received optimal dose of 18-20 mg/kg, 16 (31.3%) received supra-normal,
and rest three received sub-normal doses. The dose varied from 15.1 to
25 mg/kg (mean dose 20.22 mg/kg) Serum albumin and creatinine were
within normal range in all children.
Forty-five out of 51 (88%)
patients achieved seizure control after the first dose. All children
with febrile status epilepticus (n=10) were controlled after a single
dose. Two patients (with unknown etiology) had breakthrough seizures
(after 3 and 12 hours of loading dose), which were subsequently
controlled after second dose of 10 mg/kg. Thus overall, 47 (92%)
children achieved seizure control on FOS alone.
None of the
children showed any local or systemic adverse effects, even with PHT
levels in the supratherapeutic or toxic range. Two patients with
meningitis showed local cording of vein, attributed to vancomycin.
We found a weak correlation between the dose of intravenous FOS
received and the serum PHT levels, as depicted in Fig.1.
Serum PHT levels were in therapeutic range in 12 (23.5%),
supra-therapeutic in 23 (45%) and sub-therapeutic in 16 (31.3%)
children, as shown in Table I. Seizure control after
first dose was achieved in 15 out of 16 (93.7%) children with
sub-therapeutic levels, 10 out of 12 (83.3%) with therapeutic and 20 out
of 23 (86.9%) with supra-therapeutic levels. One child each in
sub-therapeutic and therapeutic levels required second dose after 3 and
12 hours, respectively.
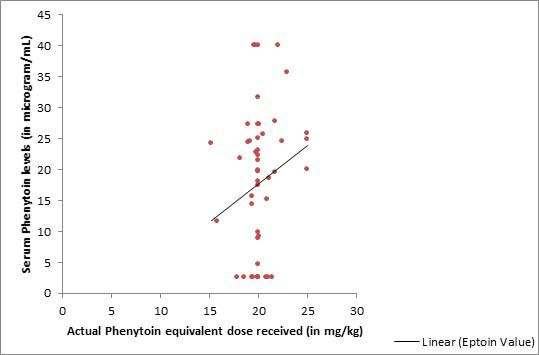 |
| Fig. 1 Correlation
of serum phenytoin levels with the actual dose of
intravevous fosphenytoin received (R2=0.038). |
Table I Serum Total Phenytoin Levels and Seizure Control in Children and Loading Dose of Fosphenytoin (N=51)
|
Dose received |
Sub- normal dose (n=3) |
Optimal dose (n=32 ) |
Supra-normal dose (n=16) | |
Phenytoin |
Very |
Low |
In |
High |
Toxic |
Very |
Low |
In |
High |
Toxic |
Very |
Low |
In |
High |
Toxic | |
levels |
low |
2.5 to |
range |
20-40 |
>40 |
low |
2.5 to |
range |
20-40 |
>40 |
low |
2.5 to |
range |
20-40 |
>40 |
|
(µg/mL) |
<2.5 |
10 |
10-20 | | |
<2.5 |
10 |
10-20 | | |
<2.5 |
10 |
10-20 | | | |
Patients, n |
1 |
- |
1 |
1 |
- |
8 |
3 |
7 |
11 |
3 |
3 |
1 |
4 |
7 |
1 | |
Seizure control achieved | |
Yes |
1 |
- |
1 |
1 |
- |
8 |
3 |
6 |
10 |
1 |
2 |
1 |
3 |
7 |
1 | |
No |
0 |
- |
0 |
0 |
- |
0 |
0 |
1* |
1* |
2# |
1* |
0 |
1 |
0 |
0 | |
Sub-normal dose: <18 mg/kg; Optimal dose: 18-20 mg/kg; Supra-normal dose: >20 mg/kg; *Needed second dose; #Needed continuous midazolam infusion. |
DISCUSSION
The
present study revealed good efficacy of IV FOS (with a dose of
18-20 mg/kg) in controlling status epilepticus in children,
similar to findings of other studies [7,8,10,11]. However, in an
African study, seizures were controlled in only 36% and 44%
patients who received PHT and FOS, respectively each in the dose
of 18 mg/kg [9].
In our study, intravenous FOS
demonstrated an excellent safety profile, even among those with
PHT levels in supratherapeutic or toxic range in 23 (45%)
patients. As compared to PHT, IV FOS has lesser rates of venous
irritation, mechanical ventilation and use of inotropic agents
[11-13]. However, in adults, 29 cardiac events, including ten
deaths were reported between 1997 to 2003, although many of
these patients had pre-existing cardiac pathology [14].
In our study, only 35 patients (68.62%) achieved PHT levels in
therapeutic or supratherapeutic range. Single-dose is shown to
achieve and maintain therapeutic levels up to 24 hours after
loading dose, irrespective of body mass index [8,15-17]. One
study suggested 22.5 mg/kg may be a better dose to achieve
therapeutic levels in children [18]. Ogutu, et al. [9] showed
comparable serum levels when intravenous FOS and PHT were given
(dose 18 mg/kg) and FOS achieved peak levels faster (mean 0.08
hours) as compared to 0.37 hours for PHT.
We found a
weak correlation between the FOS dose and PHT levels achieved.
Selioutski, et al. [19] also found similar results, 63% of those
receiving 15-20 mg/kg dose and 51% of those receiving 20-55
mg/kg dose did not achieve levels of 20 µg/mL or more within the
first 6 hours; while some patients achieved levels of >20 µg/mL
despite receiving low doses.
Therapeutic drug monitoring
of PHT levels is considered necessary to ensure non-toxic
levels, and should preferably be done at least one hour after
loading [6]. In our study, seizure control did not depend on
serum PHT levels. Also, a low incidence of adverse effects even
with blood levels in toxic range is reassuring. Thus, we did not
find any additional benefit of monitoring PHT levels, though
numbers are small. Seizure control (without adverse effects) may
be a better measure of clinical efficacy as compared to blood
levels, which indicate pharmacokinetic efficacy.
Due to
limited funding, our sample size was small, and PHT levels could
not be repeated at later time intervals to ascertain whether
they remain in the therapeutic range. We did not exclude
patients who were already on other anti-epileptic drugs before
admission, which can influence PHT levels.
In future
studies, serum PHT levels can be serially measured at different
time/points after the loading dose. Efficacy, tolerability, and
pharmacokinetics of intramuscular loading dose of FOS should
also be studied, along with a detailed pharmaco-economic
assessment.
A single loading dose of intravenous FOS
(18-20 mg/kg) is effective in controlling status epilepticus in
88% of children with very low risk of adverse events. It should
be preferred over PHT as second-line drug for status
epilepticus. Serum PHT levels were in therapeutic and
supratherapeutic range in only 68.6% at 90-100 minutes of
loading, and appear to be independent of dose received.
Acknowledgements: Mr. Srivallabh Sane (Statistician- Department
of Community Medicine), Dr. Bhakti Sarangi (PICU Incharge),
Bharati Vidyapeeth Deemed University Medical College, Pune.
Contributors: KS: conceptualized and planned the study,
along with manuscript writing; SB, VG, RP: carried out the data
collection and analysis. SR: revised the manuscript.
Funding: Institutional (Bharati Medical Foundation).
Competing interest: None stated.
What is Already Known?
•
Fosphenytoin shows good efficacy in control of seizures,
with less risk of adverse effects.
What This Study Adds?
• Fosphenytoin
showed good efficacy in children with status
epilepticus, with good safety profile.
• Serum
total phenytoin levels at 90-100 minutes showed poor
correlation with the dose of fosphenytoin received.
|
REFERENCES
1. Mishra
D, Sharma S, Sankhyan N, Konanki R, Kamate M, Kanhere S, et al.
Consensus guidelines on management of childhood convulsive
status epilepticus. Indian Pediatr. 2014;51:975-90.
2.
Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge
J, et al. Evidence-based Guideline: Treatment of Convulsive
Status Epilepticus in Children and Adults: Report of the
Guideline Committee of the American Epilepsy Society. Epilepsy
Currents. 2016;16:48-61.
3. Poplawaska M, BorowiczK,
Czuczwar SJ. The safety and efficacy of Fosphenytoin for the
treatment of status epilepticus. Expert Rev Neurother. 2015;
15:983-92.
4. Kirschbaum K, Gurk-Turner C. PHT vs
Fosphenytoin. BUMC (Baylor University Medical Center)
Proceedings. 1999;12;168-72.
5. von Winckelmann SL, Spriet I,
Willems L. Therapeutic drug monitoring of phenytoin in
critically ill patients. Pharmaco-therapy. 2008;28:1391-400.
6. McCluggage LK, Voils SA, Bullock MR. Phenytoin toxicity
due to genetic polymorphism. Neurocrit Care. 2009;10:222.
7. Allen FH, Jr, Runge JW, Legarda S. Safety, tolerance, and
pharmacokinetics of intravenous fosphenytoin (Cerebyx) in status
epilepticus. Epilepsia. 1995;36:90.
8. Moffett, Brady S,
Weingarten, Mindi M, Schmees, Lindsay R, et al. Fosphenytoin
population pharmacokinetics in the acutely ill pediatric
population. Pediatric Critical Care Med. 2018;19:748-54.
9. Ogutu BR, Newton CR, Muchohi SN, Otieno GO, Edwards G,
Watkins WM, et al. Pharmacokinetics and clinical effects of
phenytoin and fosphenytoin in children with severe malaria and
status epilepticus.Br J Clin Pharmacol. 2003;56:112-9.
10. Boucher BA, Feler CA, Michie DD, Tipton BK, Smith KR Jr,
Kramer RE, et al. The safety, tolerability and pharmacokinetics
of fosphenytoin after intramuscular and intravenous
administration in neurosurgery patients. Pharmacotherapy.
1996;16:638-45.
11. Nishiyama M, Nagase H, Tomioka K,
Tanaka T, Yamaguchi H, Ishida Y, et al. Fosphenytoin vs.
continuous midazolam for pediatric febrile status epilepticus.
Brain Dev. 2018;40:884-90.
12. Fischer JH, Patel TV,
Fischer PA. Fosphenytoin: Clinical pharmacokinetics and
comparable advantages in the acute treatment of seizures. Clin
Pharmacokinet 2003;42:33-58.
13. Jamerson BD, Dukes GE,
Brouwer KLR, Dorm KH. Venous irritation related to intravenous
administration of phenytoin versus fosphenytoin.
Pharmacotherapy. 1994; 14:47-52.
14. Adams BD, Buckley
NH, Kim JY, Tipps LB. Fosphenytoin may cause hemodynamically
unstable bradydysrhythmias. J Emerg Med. 2006;30:75.
15.
Messinger MM, Moffett BS, Wilfong A. Impact of body habitus on
Phenytoin levels following Fosphenytoin loading dose in
pediatric patients. Ther Drug Monit. 2015;37:772-5.
16.
Prusakoy AB, Patel AD, Ciole JW. Impact of obesity on
Fosphenytoin volume of distribution in pediatric patients. J
Child Neurol. 2018;33:534-36.
17. Kim DW, Kim TE, Ji M,
Chun Yi. Safety, tolerability and pharmacokinetics of
Fosphenytoin loading in patients with subarachnoid hemorrhage.
Clin Neuropharmacol. 2015;38: 248-51.
18. Tanaka J,
Kasai H, Shimizu K, Shimasaki S, Kumagai Y. Population
pharmacokinetics of PHT after intravenous administration of
Fosphenytoin sodium in pediatric patients, adult patients and
healthy volunteers. Eur J Clin Pharmacol. 2013;69:489-97.
19. Selioutski O, Grzesik K, Vasilyeva ON, Hilmarsson A,
Fessler J, Lin L, et al. Evaluation of phenytoin serum levels
following a loading dose in the acute hospital setting. Seizure.
2017;52:199-204.
|
|
|
 |
|

