|
|
|
Indian Pediatr 2019;56:
249-250 |
 |
Fecal Calprotectin as a Screening Marker for Inflammatory
Bowel Disease
|
|
Mathrubootham Sridhar* and Dhanasekhar Kesavelu
Department of Pediatrics, Apollo Children’s Hospital,
Chennai, India.
Email:
[email protected]
|
|
We compared fecal calprotectin and
endoscopic findings of 53 children with possible inflammatory bowel
disease and found an optimal cut-off of 68 µg/g in Receiver operative
curve [AUC 0.88 (95% CI 0.79, 0.97)] to discriminate inflammatory bowel
disease with other inflammatory gastrointestinal conditions.
Keywords: Crohn’s disease, Diagnosis, Ulcerative colitis.
|
|
Diagnosis of Inflammatory bowel disease (IBD) is
confirmed by clinical evaluation and a combination of endoscopic,
radiological, histological investigations. Non-invasive biomarker such
as fecal calprotectin, which is released during times of cell
stress/damage, it is a highly sensitive marker of intestinal
inflammation, and represents a novel and under-utilized modality to aid
in diagnosis of IBD. Growing body of literature has identified fecal
calprotectin (FCP) as a non-invasive predictive test with high
sensitivity for inflammatory bowel disease.
This cross-sectional study was done over a period of
one year in Apollo Children’s Hospital, Chennai, a tertiary referral
center in Southern India. Ethics approval was obtained from Institute
Ethics Committee, and informed consent of participants was obtained. We
tested 53 consecutive patients (mean (SD) age 9.7(4) years), who
presented with inflammatory bowel disease symptoms as per European
Crohn’s and Colitis Organization guidelines [1,2]. The presenting
complaints necessitating FCP testing and colonoscopy/endoscopy were:
chronic abdominal pain (52, 98.1%), chronic diarrhea (51, 96.2%), mucoid
stools (38, 71.7%), blood in stools (28, 52.8%), prolonged fever (6,
11.3%), pallor (9,17%), oral ulcers (4, 7.5%), glossitis (2, 3.8%) and
angular cheilitis (2, 3.8%). Many patients presented with combination of
symptoms mentioned above.
FCP using enzyme-linked immunosorbent assay by
LIAISON Calprotectin Assay
(negative <6.2 mg/kg) was performed at baseline for all enrolled
patients along with radiological investigations as deemed appropriate.
Colonoscopy along with endoscopy was performed on all patients the
subsequent day before the results of the FCP were available and a final
confirmation with tissue biopsy reports was done for diagnosis of
inflammatory bowel disease.
Of the 53 children, 17% had biopsy-confirmed
diagnosis of inflammatory bowel disease; eight (15.1%) had Crohn’s
disease and one (1.9%) had Ulcerative colitis. Receiver Operating
Characteristics (ROC) curve revealed an optimal cut-off for FCP level of
68 mg/kg to discriminate between IBD and non-IBD causes of inflammation
and this value had sensitivity of 100%, specificity of 70%, positive
predictive value of 40%, negative predictive value of 100%, and
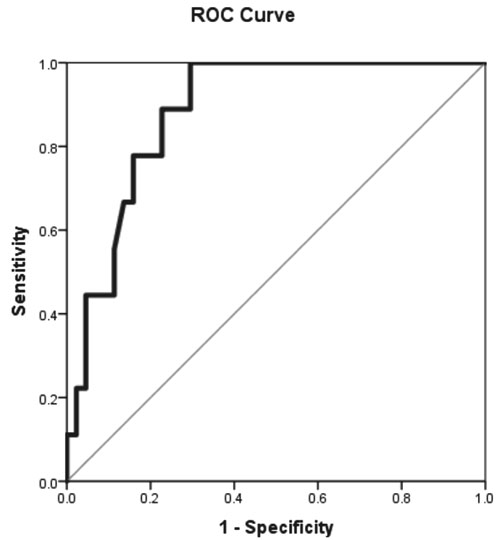
likelihood ratio for a positive test of 3.4. The area under the curve of
the ROC was 0.88 (95% CI 0.79-0.97) (Fig. 1). In our
cohort of 53 patients, by considering FCP cut-off of 68 mg/kg, we had
nine True Positives, 13 False Positives, no False Negative, and 31 True
Negatives. Mean (SD) FCP in false positive cases was 502.7 (469) µg/g
and Mean (SD) FCP among IBD cases was 818.8 µg/g (873.2).
 |
|
Fig. 1 ROC curve showing area under
the curve of 0.88 (95% CI 0.79-0.97) for FCP value of 68 mg/kg.
|
Studies with larger cohort from published literature
suggest the usefulness of calprotectin as a screening marker for
inflammatory bowel disease [3,4]. In IBD, FCP is often used to predict
mucosal healing while the patient is on therapy [1]. Endoscopy and
histology remain the current gold standard method for detecting and
monitoring bowel inflammation. A meta-analysis by Rheenen, et al.
[5] suggested FCP to be a useful screening tool for patients who were
most likely to need endoscopy for suspected IBD. Raised FCP may be
associated with other inflammatory conditions apart from inflammatory
bowel disease like rectal polyp, non-specific colitis and eosinophilic
procto-colitis [6].
We had few limitations in our study. There were no
controls. Though the levels of FCP were not known before doing the
endoscopy, we had a positive FCP in all cases, and the histopathology
showed inflammatory changes in entire cohort; though not necessarily IBD
changes. We did not have patients with negative FCP results, rendering
our study not useful to evaluate its role in all gastrointestional
inflammatory conditions. In Indian settings, infective etiologies
causing inflammatory changes are lot more common than western population
and this might result in children going through invasive procedures
unnecessarily. Therefore, FCP should be used in conjunction with more
robust non-invasive investigations before arriving at a decision to do
endoscopy.
Our pilot study in an Indian setting adds to the
growing evidence from around the world that presence of FCP in stool
highlights the need to rule out inflammatory causes for gastrointestinal
symptoms, and higher values are associated with diagnosis of IBD.
Studies with larger cohort with control group will be needed to enhance
the usefulness of this biomarker in IBD.
Acknowledgement: Balasubramaniam Ramakrishnan,
Apollo Hospitals, Chennai, for help in statistical analysis.
Contributors: Both authors contributed to
study concept, data collection, outcome assessment, and manuscript
preparation.
Funding: None; Competing interest:
None stated.
References
1. Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths
AM, de Carpi JM, Bronsky J, et al. Management of Paediatric
Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline
from European Crohn’s and Colitis Organization and European Society of
Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr
Gastroenterol Nutr. 2018;67:257-91.
2. Levine A, Koletzko S, Turner D, Escher JC,
Cucchiara S, de Ridder L, et al. ESPGHAN Revised Porto Criteria
for the diagnosis of Inflammatory Bowel Disease in Children and
Adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795-806.
3. Quail MA, Russell RK, Van Limbergen JE, Rogers P,
Drummon HE, Wilson DC, et al. Fecal calprotectin complements
routine laboratory investigations in diagnosing childhood inflammatory
bowel disease. Inflamm Bowel Dis. 2009;15:756-9.
4. Diamanti A, Panetta F, Basso MS, Forgione A,
Colistro F, Bracci F, et al. Diagnostic work up of inflammatory
bowel disease in children: The role of calprotectin assay. Inflamm Bowel Dis. 2010;16:1926-30.
5. Van Rheenen PF, Van de Vijver E, Fidler V. Faecal
calprotectin for screening of patients with suspected inflammatory bowel
disease: diagnostic meta-analysis. BMJ. 2010;341:c3369.
6. Kelsen J, Baldassano RN. Inflammatory bowel disease: The
difference between children and adults. Inflamm Bowel
Dis. 2008;14:S9-11.
|
|
|
 |
|

