|
|
|
Indian Pediatr 2019;56: 244-24 6 |
 |
Posterior
Reversible Encephalopathy Syndrome Complicating Diabetic
Ketoacidosis
|
|
Santhosh Olety Sathyanarayana 1,
Padmanjali K Sreenivas2
and Anil Malugonahalli Uddappa2
From 1Departments of Pediatric
Endocrine and Diabetes, Karnataka Institute of Endocrinology and
Research; and 2Department of Pediatrics, Cloudnine
Children’s Hospital; Bengaluru, Karnataka, India.
Correspondence to: Dr Santhosh Olety Sathyanarayana,
Consultant Paediatric Endocrine and Diabetes, Karnataka Institute of
Endocrinology and Research, Bannerghatta Road, Jayanagar 9th block,
Jayadeva Cardiology Hospital and Research Campus, Bengaluru 560 009,
Karnataka, India.
Email: [email protected]
Received: July 28, 2017;
Initial review: January 01, 2018;
Accepted: December 18, 2018.
|
Background: Posterior reversible encephalopathy syndrome (PRES) is a
benign disorder of reversible subcortical vasogenic cerebral edema.
Case characteristics: A 13-yr-old girl presented 4 days after
complete recovery from diabetic ketoacidosis with symptoms of headache,
altered sensorium, seizures, and visual loss. There was no hypertension
or biochemical abnormalities identified. MRI brain showed hyperintense
areas in subcortical and periventricular white matter of bilateral
fronto-parieto-occipetal lobes, with possible diagnosis of normotensive
PRES. Outcome: Full recovery without sequelae, following neuro-protection
and expectant treatment. Message: Identifying PRES in diabetic
ketoacidosis assists appropriate treatment and prognostication.
Key words: Hypertension, Leukoencephalopathy, Outcome,
Seizures.
|
|
R
eversible posterior leukoencephalopathy syndrome
(PRES) is a clinicoradiological entity characterized by headaches,
altered mental status, seizures, and visual loss and is associated with
white matter vasogenic edema predominantly affecting the posterior
occipital and parietal lobes of the brain [1]. Many possible triggers
including hypertension, impaired renal function, immunosuppressive
therapies and various inflammatory conditions were seen [2], but it can
occur with many diverse clinical entities. The diagnosis can be made
clinically and is reinforced by characteristic changes observed on MRI
brain. Typical MRI findings include reversible, symmetrical, posterior
subcortical vasogenic edema [1]. If recognized and treated promptly, the
rapid-onset symptoms and radiologic features usually fully resolve
within days to weeks. [3]
Case Report
A previously well 13-year-old girl presented with DKA
as a first presentation of Type 1 diabetes mellitus (T1DM). Initially
she was managed in a different hospital and found to have blood glucose
of 510 mg/dL, severe dehydration, heart rate of 140/minute,
saturation 90% in air, blood pressure 100/70 mmHg with peripheral
perfusion of 2 secs and metabolic acidosis. Over the course of 12 hours
in the referring hospital, she had received 2800 mL of fluids.
She had urine output at 5 mL/kg/hr. Cerebral odema was suspected in view
of deteriorating Glasgow coma scale, CO 2
retention and persisting severe acidosis. She received a dose of
mannitol, intubated, ventilated and transferred to our centre. CT
brain reported normal and cerebral edema was ruled out. She was
extubated within 24 hours and recovered from DKA within 48 hours but
found to have lower limb weakness and hypotonia with grade 3 power. No
obvious identifiable cause was found for weakness except for raised
creatine phosphokinase (742 U/L) which normalised within a week. She is
the only child and mother is known case of Dermatosclerosis.
Biochemistry on day 1 indicated severe intravascular
fluid depletion with serum sodium (Na) 162 mmol/l, serum potassium (K)
2.3 mmol/l, serum creatinine (Cr) 1.2 mg/dL and blood urea 32 mg/dL; all
of these normalized by 3 days. On day 5, she was discharged on basal
bolus insulin regimen with blood pressure recorded 119/70 mmHg and blood
glucose 226 mg/dL.
She presented again to triage 4 days post-discharge
with complaints of being drowsy, blurring of vision, headache and two
episodes of seizures lasting for 30 seconds, described as vacant stare
with eyes rolled up, increased tone of all four limbs, twitching of
angle of mouth and right eye, with blood glucose 109 mg/dL, pulse rate
112/min and BP 127/80 mmHg (90 th
centile) at presentation. She received 20 mg/kg (PE) loading dose of
fosphenytoin. She remained drowsy and confused with heart rate 98/min,
peripheral pulses feeble, respiratory rate 16/min with poor respiratory
efforts, SpO2 87% on room
air and 98% on 3L oxygen. Hence she was electively intubated, VBG and
biochemistry post intubation showed pH 7.3, PCO2
58 mmHg, bicarbonate 28.5 mmol/l, Na 144 mmol/L, K 3.5 mmol/L, Cl 85 meq/L
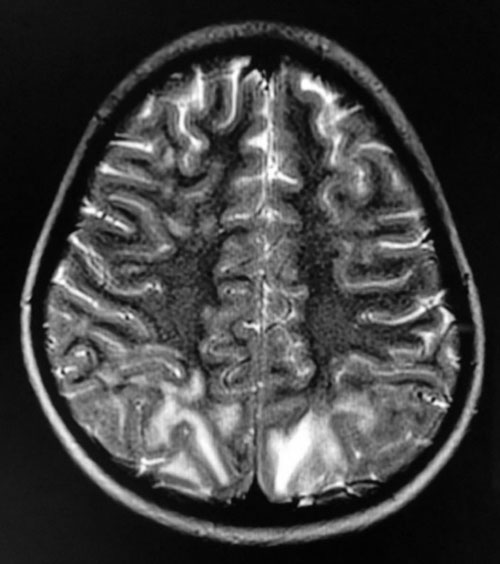
and Cr 0.5 mg/dL. MRI brain showed hyperintense areas in subcortical
white matter of bilateral parieto-occipital lobes and no diffusion
restriction (Fig. 1) with possible diagnosis
of PRES. She got extubated after 3 days, blood pressure remained around
124/80 mmHg, vasculitis profile and infection screening (CSF, blood,
urine cultures) was negative. Over the following two weeks, her lower
limb weakness and vision disturbances improved completely. At last
follow-up, two years after discharge, she remains well without any
neurological symptoms and her HbA1C has been less than 6% (43 mmol/mol).
 |
|
Fig. 1 MRI brain T2 sequence showing
hyperintense areas in subcortical white matter of bilateral
occipital lobes.
|
Discussion
The child developed progressive encephalopathy,
cortical blindness and seizures, typical of PRES and the same supported
by characteristic appearances on MRI. As anticipated her clinical
deficits improved rapidly with expectant management. The pathophysiology
in PRES is the vasogenic oedema which has a favorable outcome when
compared to osmotic cerebral edema seen in DKA [4]. Osmotic edema occurs
during the course of DKA and is a leading cause of morbidity and
mortality in children with type 1 diabetes [5], whereas vasogenic odema
is usually seen after recovery from DKA as in our case.
The underlying pathogenesis of PRES is not fully
understood, but is thought to be caused by endothelial damage or
dysfunction caused by excessive circulating inflammatory cytokines
[4,6]. This is important because DKA is associated with increased serum
proinflammatory cytokines [7], shown to up regulate expression of
vascular endothelial growth factor and may therefore increase vascular
permeability in PRES [4,8].
Hypertension and BP flctuations are recognized as
potential triggers in PRES [1,2] but cases have been reported with
normotensive PRES as well [3]. Electrolyte disturbances may have been
contributory.
Hypertension and blood pressure fluctuations were not
noticed in this child, unlike a common finding in the cases reported
previously. Electrolyte disturbances such as hypokalaemia, hypernatremia
in our case may have been contributory. The brain edema frequently
involves, parieto-occipital pattern; though, it may also involve more
anterior regions [4]. Nearly, half of the patients who develop PRES have
a history of autoimmune diseases (e.g. systemic lupus
erythematosus, hypothyroidism, Crohn’s disease) [4], but there has been
a recent report associated with onset of T1DM complicated by DKA [9].
Our case could be the second one reported showing association of PRES
following DKA in children. Development of PRES in this patient was
likely to be multifactorial and may have been potentiated by the
metabolic effects of DKA and postulated indirect effects of electrolyte
disturbances on vascular permeability.
Since PRES is often unsuspected by clinicians,
recognizing the characteristic image findings by radiologists is key in
diagnosing this syndrome and should guide the clinicians in preventing
and minimizing deleterious work-ups or therapies unless there is
atypical presentation. One can expect an excellent clinical outcome
within few days.
Acknowledgements: Dr HK Anand and Dr BP Pooja,
Radiologist, Medall Clumax Diagnostics, for their expertise with MRI
images.
Contributors: OSS: drafted and edited the
manuscript; AMU, PS: assisted in data collection, drafting and managing
the case in PICU.
Funding: None; Competing interest: None
stated.
References
1. McKinney AM, Short J, Truwit CL, McKinney ZJ,
Kozak OS, Santa Cruz KS, et al. Posterior reversible
encephalopathy syndrome: incidence of atypical regions of involvement
and imaging findings. Am J Roentgenol. 2007;189:904-12.
2. Roth C, Ferbert A. The posterior reversible
encephalopathy syndrome: What’s certain, what’s new? Pract
Neurol. 2011;11:136-44.
3. Hinchey J, Chaves C, Appignani B, Breen J, Pao
L, Wang A, et al. A reversible posterior leukoencephalopathy
syndrome. N Engl J Med.1996;334:494-500.
4. Fugate JE, Rabinstein AA. Posterior reversible
encephalopathy syndrome: Clinical and radiological manifestations,
pathophysiology, and outstanding questions. Lancet Neurol.
2015:14:914-25.
5. Sperling MA. Cerebral edema in diabetic
ketoacidosis: an underestimated complication? Pediatr Diabetes.
2006;7:73-4.
6. Bartynski W. Posterior reversible encephalopathy
synd-rome, part 2: Controversies surrounding pathophysiology of
vasogenic edema. Am J Neuroradiol. 2008;29:1043-9.
7. Hoffman WH, Burek CL, Waller JL, Fisher LE, Khichi
M, Mellick LB. Cytokine response to diabetic ketoacidosis and its
treatment. Clin Immunol. 2003;108: 175-81.
8. Isales CM, Min L, Hoffman WH. Acetoacetate and
betahydroxybutyrate differentially regulate endothelin 1 and vascular
endothelial growth factor in mouse brain microvascular endothelial
cells. J Diabetes Complications. 1999;13:91-7.
9. Jones R, Redler K, Witherick J, Fuller G, Mahajan
T, Wakerley BR. Posterior reversible encephalopathy syndrome
complicating diabetic ketoacidosis; An important treatable complication.
Pediatric Diabetes. 2017;18:159-62.
|
|
|
 |
|

