D glucan is a
panfungal marker (unlike the GM test which detects only IA) present in
all fungi except Mucormycetes and Cryptococcus. There are
four commercial kits available. The only FDA approved kit is Fungitell.
The other three kits (Fungitec-G, Wako, Maruha) are mostly used
in Japan. A cut-off value of <60 pg/mL is considered as negative, >80
pg/mL as positive and 60-80 pg/mL as borderline in adults with Fungitell.
False positive may be seen with bacteremia with gram-positive or
negative bacteria, mucositis and mucosal colonization with Candida
species, administration of albumin and immunoglobulins, thrombocyte
infusion with leukocyte removing filters, hemodialysis with cellulose
membranes, use of blood products filtered through cellulose filters,
P. jirovecii infection, administration of meropenem, cefepime,
piperacillin tazobactam, or amoxicillin-clavulanate [26-29]. It has been
observed that BDG levels are higher in healthy children as compared to
healthy adults [30]. At present, the diagnostic utility of BDG in
children is limited with poorly established cut-offs. It might help
either as an adjuvant with other diagnostic methods or as an indirect
parameter to monitor IFIs.
Mannan antigen (Mn) and anti-Mannan antibody (a-Mn):
Detection of mannan antigen may be helpful in serum of hepatosplenic
candidiasis and Candida meningitis where blood culture is rarely
positive [31]. The sensitivity and specificity of Mn/a-Mn antibodies
combination is 83% and 86%, respectively. The sensitivity depends on
type of Candida spp (species specific) viz 100% in
Candida albicans, 50% in Candida krusei and Candida kefyr
and 40% in Candida parapsilosis [32]. Presently, utility of
this assay is limited because of several disadvantages like rapid
clearance of the Candida Mn from serum, and cross-reactions due
to colonization with Candida spp.
Markers for Cryptoccocosis and Histoplasmosis:
Diagnosis of Cryptococcal meningitis or disseminated cryptococcosis is
possible by demonstration of encapsulated yeast cells on India ink,
culture or detection of Cryptococcal antigen (CrAg). The detection of
CrAg in serum or CSF can be done by latex agglutination test (LA) and
enzyme immunoassay (EIA). The sensitivity and specificity of LA varies
from 93%-100% and 93%- 98 %. False-positive findings have been reported
in cases of Trichosporon spp., Capnocytophaga spp., or
Stomatococcus spp. infections or detergents. Recently,a point of
care assay, an immune chromatographic lateral flow assay (CrAg LFA;
Immuno-Mycologics, Norman, OK, USA) has been designed which has a
sensitivity and specificity of 98% [21]. It is cheaper, has shorter
turnaround time (15 min) and can also detect C. gattii, an
additional advantage in comparison to other CrAg tests available [33].
Histoplasma takes several weeks to grow on culture.
Detection of Histoplasma antigen from serum or urine is non-invasive,
and is a rapid test for diagnosis in disseminated histoplasmosis. The
sensitivity is highest in disseminated histoplasmosis followed by acute
and chronic pulmonary and least in subacute pulmonary histoplasmosis.
Rising antigen levels can also be used as early predictors for clinical
relapse or treatment failure. A limitation of antigen testing is the
significant cross-reactivity of the assay in the presence of other
fungal infections, including blastomycosis, paracoccidioido-mycosis,
penicilliosis, aspergillosis, and coccidio-idomycosis [34].
Molecular Methods
Molecular methods are potential alternative options
for early diagnosis of IFI, ideal being broad-range/pan-fungal PCR.
European Fungal PCR Initiative group (FPCRI) have standardized and
validated protocols for Aspergillus PCR which can be used as a
screening tool because of its high negative predictive value. SeptiFast
PCR, a commercial mutiplex realtime PCR (Roche diagnostics, Germany)
available for 20 clinically relevant pathogens including six fungi
i.e. five Candida spp and A. fumigatus proved to be
helpful where culture was negative. Rate of positivity was 14.6% as
compared to culture (10.3%) [35]. PCR positivity depends on site and
amount of clinical specimen. The Asper Genius assay detects and
differentiates wild type from pathogenic Aspergillus fumigatus
with (four) azole resistance associated mutations in the cyp51A
gene. FKS1- echinocandin resistance for Candida spp can also be
done directly from blood [36].
Recently FDA approved T2 candida method which is a
rapid test to detect five species of Candida (C. albicans, C.
tropicalis, C. parapsilosis, C. krusei and C. glabrata) directly
from blood sample. Principle of the assay is initial nucleic acid
extraction amplification and hybridization of the product. The detection
limit is 1 CFU/ml (compared 100 -1000 CFU/ml in NAATs). It is fully
automated and results provided within 3-5 hours. However, this technique
has not yet been evaluated in pediatric population [21].
Radiological Diagnosis
The role of imaging in invasive fungal infections is
multi-dimensional. Imaging helps in identification of the focus of
infection, establishing a possible diagnosis, and detection of serial
changes. Plain radiographs often prove inconclusive. Other imaging
modalities include computed tomography (CT), ultrasound and magnetic
resonance imaging (MRI).
Contrast enhanced CT scan remains the most important
imaging modality in the diagnosis of invasive fungal infections.
Evaluation of the pulmonary pathology requires a reconstruction of
images into routine lung window and high resolution lung window images.
With the present day fast CT scanners and a volumetric acquisition of
data, good quality image acquisition in a very short time frame is made
possible. This is especially crucial in acutely ill febrile or dyspneic
children. The appearance of new abnormalities on CT chest not responding
to broad spectrum antibiotics should be considered as possible IFI in
these children.
Detection of any abdominal focus of infection
requires a meticulous ultrasound examination with additional high
resolution ultrasonography, using high frequency linear transducer of
5-12 MHz, of the solid organs such as liver, spleen and
kidneys.Transcranial USG using a small footprint sector probe is useful
in neonates and young infants.
MRI is invaluable in the evaluation of infection of
the central nervous system and musculoskeletal system. MRI is helpful in
follow-up of pulmonary fungal infections as it is non-ionizing
radiation, though its sensitivity in the detection of small pulmonary
nodules in infection is less than CT.
CNS infections: CNS infections in IFI vary
depending on the organism. CNS aspergillosis may be seen on imaging as (i)
multiple areas of embolic infarcts secondary to meningitis and
involvement of perforating vessels, (ii) multiple ring enhancing
lesions or (iii) contiguous involvement of the dura associated
with adjacent sinusitis/skull base osteomyelitis [37]. CNS mucor-mycosis
is usually associated with sinusitis and bone erosion. Often there is
associated cavernous sinus thrombosis and contiguous CNS spread of
infection (Web Fig. 1). Disseminated hematogenous candida
infection may result in meningoencephalitis. The imaging findings
include meningeal enhancement and multiple ring enhancing or nodular
lesions in the brain parenchyma.
Screening patients with probable and proven invasive
pulmonary aspergillosis by MRI of brain is recommended even in absence
of neurologic signs/symptoms. Dissemination of pulmonary aspergillosis
to CNS has been reported in 14% and clinical features of CNS infection
occur late in the course of disease.
Sinonasal infection (Web Fig. 2):
Sinonasal invasive fungal infection on imaging appears as soft
tissue opacification of the sinonasal cavity with mucoperiosteal
thickening; with variable degree of bony erosion. Skull base
osteomyelitis and contiguous CNS spread of infection can occur as a
complication.
Pulmonary infection: Pulmonary candidiasis can
present as a part of disseminated candidiasis. The imaging features can
manifest as lobar consolidation and mimic bacterial pneumonia or may
present as multiple nodules similar to aspergillosis. Invasive
aspergillosis (Web Fig. 3) usually shows
multiple pulmonary nodules often with perinodular ground glass opacity
‘halo sign’. The imaging signs of improvement include cavitation
(crescent sign) within the nodule. Pulmonary mucormycosis can present as
airspace consolidation or multiple nodules. The nodules may show halo
sign or reverse halo sign. Reverse halo sign refers to an area of
peripheral consolidation and a central ground glass opacity. Bird’s nest
sign is seen as central ground glass opacity with multiple intersecting
or irregular lines [38]. Both the signs can be seen in angioinvasive
aspergillosis, mucormycosis, cryptogenic organizing pneumonia, Wegener’s
granulomatosis and other causes.
Abdominal infection: Disseminated candidemia may
show focal lesions in liver, spleen and other abdominal organs. The
lesions appear hypoechoic, target lesion or as small abscesses on USG.
CT has superior sensitivity than USG in detecting focal hepatosplenic
lesions which appear hypodense (Web Fig. 4).
Musculoskeletal: Musculoskeletal involvement in
disseminated fungal infection may manifest as osteomyelitis and
arthritis. Vertebral and costal fungal osteomyelitis can develop from
contiguous pulmonary infection as in aspergillosis, or by hematogenous
dissemination and traumatic inoculation. The imaging findings are
similar to other forms of osteomyelitis and include osteopenia, erosions
and periosteal reaction.
Approach to a Suspected Case of Fungal Infection
The risk factors for occurrence of fungal infections
must be kept in mind while evaluating a patient of pyrexia of unknown
origin or unusual signs/symptoms at usual/unusual sites. If signs
/symptoms of localized fungal infection are present (oral ulcers/skin
ulcers/pneumonia/sinusitis) sample must be sent as soon as possible for
direct microscopy and culture for definitive diagnosis. The treating
team must interact with the mycologist and radiologist when evaluating
such a case. The investigations must be ordered judiciously and
interpreted rationally as definite evidence is often lacking and
antifungals have significant side effects and require prolonged
administration. They should be chosen depending on the organism
identified, host characteristics, site of infection and local
epidemiological data of fungal infections including resistance.
A suggested algorithm for evaluation of a suspected
case of fungal infection is depicted in Fig 1.
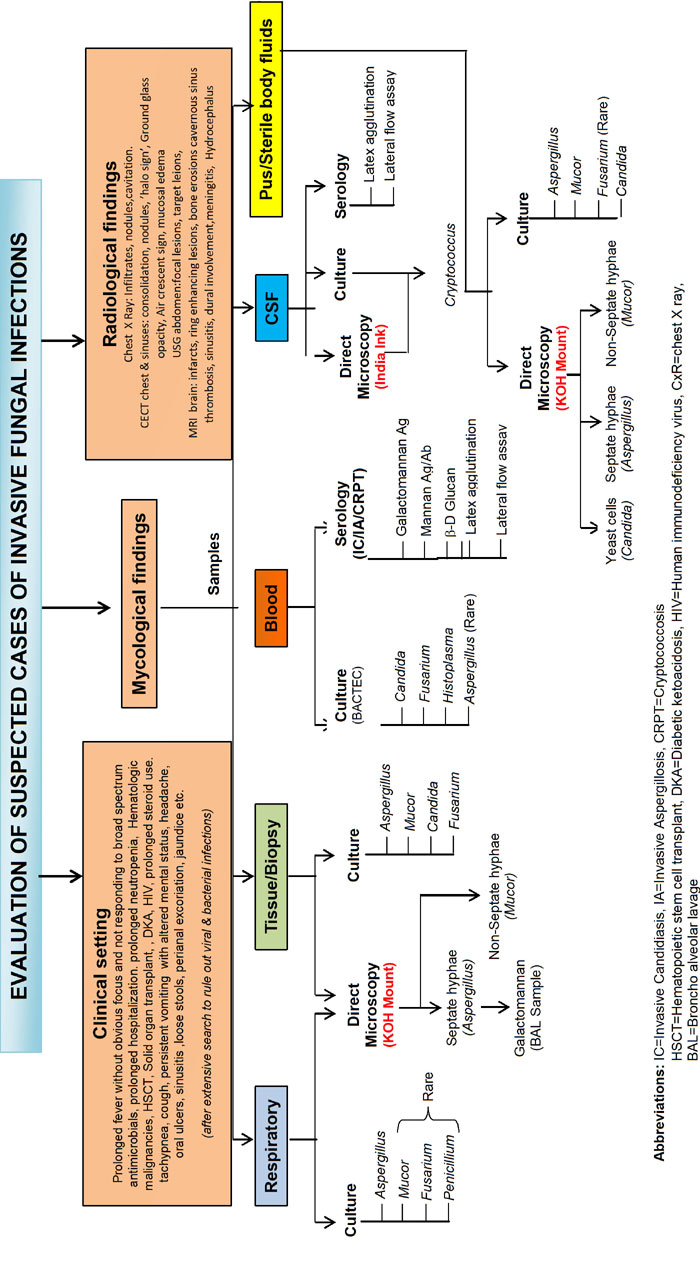 |
|
Fig. 1 Evaluation of suspected
invasive fungal infection.
|
Conclusion
Diagnosis of IFIs in children remains challenging.
The validation and utility of various currently available fungal
diagnostic tools are lacking, particularly in the pediatric group.
Although tissue diagnosis remains the gold standard, other tests like
Galactomannan assay and PCR can be used as adjunct for diagnosis of IFI
in children. Newer methods like T2 candida and lateral flow assay need
validation in children with candidemia and invasive aspergillosis. Role
of radiology in diagnosing IFI needs further exploration.
References
1. Rosen GP, Nielsen K, Glenn S, Abelson J, Deville
J, Moore TB. Invasive fungal infections in pediatric oncology patients:
11-year experience at a single institution. J Pediatric Hematol Oncol.
2005;27:135-40.
2. Wattier RL, Dvorak CC, Hoffman JA, Brozovich AA,
Bin-Hussain I, Groll AH, et al. A prospective, international
cohort study of invasive mold infections in children. J Pediatr Infect
Dis Soc. 2014;4:313-22.
3. Jordan I, Balaguer M, López-Castilla JD, Belda S,
Shuffelman C, Garcia-Teresa MA, et al. for the ERICAP Study
Group. Per-species risk factors and predictors of invasive Candida
infections in patients admitted to pediatric intensive care units:
development of ERICAP scoring systems. Pediatr Infect Dis J.
2014;33:s187-93.
4. Jaworski R, Haponiuk I, Irga-Jaworska N, Chojnicki
M, Steffens M, Paczkowski K, et al. Fungal infections in children
in the early postoperative period after cardiac surgery for congenital
heart disease: A single-centre experience. Interact Cardiovasc Thorac
Surg. 2016;23: 431-7.
5. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA,
Edwards JE, Calandra T, et al. Revised Definitions of Invasive
Fungal Disease from the European Organization for Research and Treatment
of Cancer/Invasive Fungal Infections Cooperative Group and the National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG) consensus group. Clin Infect Dis. 2008;46:1813-21.
6. W Adilia, Thomas L. Progress in the Diagnosis of
Invasive Fungal disease in Children. Curr Fungal Infect Rep.
2017;11:35-44.
7. Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum
MA. Combination treatment of invasive fungal infections. Clin Microb
Rev. 2005;18:163-94.
8. King J, Pana ZD, Lehrnbecher T, Steinbach WJ,
Warris A. Recognition and clinical presentation of invasive fungal
disease in neonates and children. J Pediatr Infect Dis Soc.
2017;6:S12-21.
9. Ascioglu S, Rex JH, De Pauw B, Bennett JE, Bille
J, Crokaert F, et al. Defining opportunistic invasive fungal
infections in immunocompromised patients with cancer and hematopoietic
stem cell transplants: An international consensus. Clin Infect Dis.
2002;34:7-14.
10. Oz Y, Kiraz N. Diagnostic methods for fungal
infections in pediatric patients: Microbiological, serological and
molecular methods. Expert Rev Anti Infect Ther. 2011;9;289-98.
11. Badiee P, Hashemizadeh Z. Opportunistic invasive
fungal infections: Diagnosis and clinical management. Indian J Med Res.
2014;139:195-204.
12. Clancy CJ, Nguyen MH. Finding the "missing 50%"
of invasive candidiasis: How nonculture diagnostics will improve
understanding of disease spectrum and transform patient care. Clin
Infect Dis. 2013;56:1284-92.
13. Hope WW, Castagnola E, Groll AH, Roilides E,
Akova M, Arendrup MC, et al. ESCMID Guideline for the Diagnosis
and Management of Candida Diseases 2012: Prevention and Management of
Invasive Infections in Neonates and Children Caused by Candida Spp. Clin
Microbiol Infect. 2012;18:38-52.
14. Kontoyiannis DP, Sumoza D, Tarrand J, Bodey GP,
Storey R, Raad II. Significance of aspergillemia in patients with
cancer: A 10-year study. Clin Infect Dis. 2000;31:188-9.
15. Lass-Florl C, Resch G, Nachbaur D, Mayr A, Gastl
G, Auberger J, et al. The value of computed tomography-guided
percutaneous lung biopsy for diagnosis of invasive fungal infection in
immunocompromised patients. Clin Infect Dis. 2007;45:e101-4.
16. Hoenigl M, Prattes J, Spiess B, Wagner J,
Prueller F, Raggam RB, et al. Performance of galactomannan,
beta-d-glucan, aspergillus lateral-flow device, conventional culture,
and PCR tests with bronchoalveolar lavage fluid for diagnosis of
invasive pulmonary aspergillosis. J Clin Microbiol. 2014;52:2039-45.
17. Skiada A, Lass-Floerl C, Klimko N, Ibrahim A,
Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of
mucormycosis. Med Mycol. 2018;56:93-101.
18. Klont RR, Mennink-Kersten MA, Verweij PE. Utility
of aspergillus antigen detection in specimens other than serum
specimens. Clin Infect Dis. 2004;39:1467-74.
19. Leeflang MM, Debets Ossenkopp YJ, Wang J, Visser
CE, Scholten RJ, Hooft L, et al. Galactomannan detection for
invasive aspergillosis in immunocompromised patients. Cochrane Database
of Syst Rev. 2015;4:CD007394.
20. Groll AH, Castagnola E, Cesaro S, Dalle JH,
Engelhard D, Hope W, et al. Fourth European conference on
infections in leukaemia (ECIL-4): Guidelines for diagnosis, prevention,
and treatment of invasive fungal diseases in paediatric patients with
cancer or allogeneic haemopoietic stem-cell transplantation. Lancet
Oncol. 2014;15:e327-40.
21. Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM,
Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive
fungal infections. Clin Microbiol Rev. 2014;27:490-526.
22. Mennink-Kersten MA, Ruegebrink D, Klont RR,
Warris A, Gavini F, Op den Camp HJ, et al. Bifidobacterial
lipoglycan as a new cause for false-positive platelia Aspergillus
enzyme-linked immunosorbent assay reactivity. J Clin Microbiol.
2005;43:3925-31.
23. Pana ZD, Vikelouda K, Roilides E. Diagnosis of
invasive fungal diseases in pediatric patients. Expert Rev Anti Infect
Ther. 2016;14:1203-13.
24. Thornton CR. Development of an immuno-chromatographic
lateral-flow device for rapid serodiagnosis of invasive aspergillosis.
Clin Vaccine Immunol. 2008;15:1095-105.
25. Pan Z, Fu M, Zhang J, Zhou H, Fu Y, Zhou J.
Diagnostic accuracy of a novel lateral-flow device in invasive
aspergillosis: a meta-analysis. J Med Microbiol. 2015;64:702-7.
26. Desmet S, Van Wijngaerden E, Maertens J,
Verhaegen J, Verbeken E, De Munter P, et al. Serum (1-3)-beta-D-glucan
as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients
with human immunodeficiency virus infection or hematological malignancy.
J Clin Microbiol. 2009;47:3871-4.
27. Mennink-Kersten MA, Ruegebrink D, Verweij PE.
Pseudomonas aeruginosa as a cause of 1,3-beta-D-glucan assay reactivity.
Clin Infect Dis. 2008;46:1930-1.
28. Marty FM, Lowry CM, Lempitski SJ, Kubiak DW,
Finkelman MA, Baden LR. Reactivity of (1->3)-beta-d-glucan assay with
commonly used intravenous antimicro-bials. Antimicrob Agents Chemother.
2006;50:3450-3.
29. Usami M, Ohata A, Horiuchi T, Nagasawa K,
Wakabayashi T, Tanaka S. Positive (1->3)-beta-D-glucan in blood
components and release of (1->3)-beta-D-glucan from depth-type membrane
filters for blood processing. Transfus. 2002;42:1189-95.
30. Smith PB, Benjamin DK, Jr., Alexander BD, Johnson
MD, Finkelman MA, Steinbach WJ. Quantification of 1,3-beta-D-glucan
levels in children: preliminary data for diagnostic use of the beta-glucan
assay in a pediatric setting. Clin Vaccine Immunol. 2007;14:924-5.
31. Kumar J, Singh A, Seth R, Xess I, Jana M, Kabra
SK. Prevalence and predictors of invasive fungal infections in children
with persistent febrile neutropenia treated for acute leukemia – A
prospective study. Indian J Pediatr. 2018;85:1090-5.
32. Mikulska M, Calandra T, Sanguinetti M, Poulain D,
Viscoli C. The use of mannan antigen and anti-mannan antibodies in the
diagnosis of invasive candidiasis: recommendations from the third
European conference on infections in leukemia. Crit Care. 2010;14:R222.
33. Vidal JE, Boulware DR. Lateral flow assay for
cryptococcal antigen: An important advance to improve the continuum of
HIV care and reduce cryptococcal meningitis-related mortality. Rev Inst
Med Trop Sao Paulo. 2015;57:38-45.
34. Azar MM, Hage CA. Laboratory diagnostics for
histoplasmosis. J Clin Microbiol. 2017;55:1612-20.
35. Warhurst G, Maddi S, Dunn G, Ghrew M, Chadwick P,
Alexander P, et al. Diagnostic accuracy of SeptiFast
multi-pathogen real-time PCR in the setting of suspected
healthcare-associated bloodstream infection. Intensive Care Med.
2015;41:86-93.
36. Chong GL, van de Sande WW, Dingemans GJ,
Gaajetaan GR, Vonk AG, Hayette MP, et al. Validation of a new
Aspergillus real-time PCR assay for direct detection of Aspergillus and
azole resistance of Aspergillus fumigatus on bronchoalveolar lavage
fluid. J Clin Microbiol. 2015;53:868-74.
37. Ashdown BC, Tien RD, Felsberg GJ. Aspergillosis
of the brain and paranasal sinuses in immunocompromised patients: CT and
MR imaging findings. Am J Roentgenol. 1994;162:155-9.
38. Georgiadou SP, Sipsas NV, Marom EM, Kontoyiannis DP. The
diagnostic value of halo and reversed halo signs for invasive mold
infections in compromised hosts. Clin Infect Dis. 2011;52:1144-55.

