|
|
|
Indian Pediatr 2019;56: 202-204 |
 |
Association of TLR4 and TNF- a
Gene Polymorphisms and TLR4 mRNA Levels in Preterm Birth in a
Northern Indian Population
|
|
Shally Awasthi and Monika Pandey
From Departments Pediatrics, King George’s Medical
College University, Lucknow, Uttar Pradesh, India.
Correspondence to: Dr Shally Awasthi, Department of
Pediatrics, King George‘s Medical University, Lucknow 226 003, Uttar
Pradesh, India.
Email: [email protected]
Received: December 23, 2017;
Initial review: May 04, 2018;
Accepted: December 29, 2018.
|
|
Objective: To assess the
association of TLR4 (rs4986790 and rs4986791) and TNF- a
(rs1800629) genes polymorphisms and TLR4mRNA levels with preterm birth.
Methods: Hospital-based case-control study on women of Caucasoid
morphological subtype ethnicity in Northern India. Inclusion criteria
for cases: women aged between 18-40 years with preterm birth (<37 weeks
gestation), and for controls: women who delivered a term neonate
consecutive to an enrolled case. Three polymorphisms TLR4 (Asp299
Gly, Thr399 Ill) and TNF-á (-308G/A) and TLR4 mRNA levels were
compared between cases and controls. Results: From
2012-2015, 559 cases and 559 controls were recruited. TLR4 mRNA levels
were found to be higher (P<0.001) in cases [(0.7 (0.04)] than in
controls [(0.5 (0.04)]. No association was found between TLR4
Asp299 Gly, TLR4 Thr399 Ill and TNF-a
(-308G/A) with preterm birth. Conclusion: Increased TLR4
mRNA levels seem to be associated with preterm birth, and can be
investigated further as a potential biomarker for identifying women at
risk.
Keywords: Biomarker, Inflammation, Pregnancy
complications, Pematurity.
|
|
P
reterm births contribute significantly to
neonatal mortality and childhood morbidity in developing countries [1].
Globally, the incidence of preterm birth is highest in India at 23.6%
[2]. There is a need to find etiological causes of preterm birth,
including its genetic associates and biomarkers of inflammation.
TLR-4 and TNF-a
are the strong candidate genes of inflammatory pathway. TLR-4 has
leucine rich repeats or pattern recognition receptors that help to
identify the molecular pattern of several pathogens which initiates the
activation of cascade of inflammatory pathway [2]. The objective of this
study was to assess the association of the SNP at the rs4986790 and
rs4986791 of the TLR4 gene and rs361525 of TNF-a
gene and the expression analysis of TLR-4 gene with preterm
birth.
Methods
This was a case control study conducted in two
hospitals in Lucknow (India): King George’s Medical University and Ram
Manohar Lohia Hospital. Ethical clearance was obtained from
Institutional committees of the respective hospitals. Cases were mothers
(age 18-40 years) of live preterm (<37 weeks) neonates, while controls
were eligible mothers who delivered a neonate at or after 37 weeks of
gestation, consecutive to an enrolled case. Mothers with known
clinically diagnosable causes of preterm birth, twin delivery,
congenital abnormalities or complications in pregnancy were excluded.
Clinical, anthropometry and demographic data were extracted from
hospital records. The peripheral blood was collected and genomic DNA was
extracted from the mothers after obtaining their consent.
Both the polymorphisms of TLR4, SNP rs4986790
(A+896 G) and rs4986791 (C+1196T) are located in the coding region which
further regulate the transcription of the gene. These polymorphic sites
cause the exchange of an amino acid on alteration with the rare allele:
an aspartic acid in exchange for glycine at position 299 i.e.
Asp299Gly and threonine for an isoleucine at position 399:
Thr399IIl [2]. Alternatively, TNF- a
(rs361525) SNP is located on the promoter region of the gene and is
reported to be associated with elevated expression of the gene [2]. The
details of primers, PCR condition, restriction enzymes, and their
products are given in Web Table I. Total RNA was
extracted the peripheral blood with the help of QIAGEN kit. All the
reverse transcription was carried out by Fermentas reverse transcriptase
kit using oligo dT priming.
Real-time PCR was performed with 1 µL cDNA, Syber
green universal PCR mix, and 20X primer (Applied Biosystems, Foster
City, CA), in a 7000 Sequence Detection System (ABI Prism, Applied
Biosystems).The 18S rRNA gene was used as an endogenous control. Results
were evaluated using pfaffa method: the delta-delta Ct method, where
delta Ct was calculated as (TLR4Ct - 18sRNA Ct), and the relative
quantity of TLR4 mRNA expression was calculated by the delta–delta Ct as
2 " [(case delta Ct) "
(control sample delta Ct)]
[3].
Statistical analysis: All the analyses was
carried out by SPSS (version 22.0). The genotypic and expression
analysis was done blinded toward the patient‘s status. For categorical
variables chi-square test was used. Student‘s t-test was used to
determine the association of preterm birth with other factors. We
calculated genotypic distribution by Pearson‘s
c2 test taking 95% of
confidence interval (C.I.) in consideration. P
£0.05 was considered
significant.
Results
A total of 559 cases and 559 controls were recruited.
Table I contains the demographic and anthropometric
details of neonates and mothers. Table II depicts
genotypic and allelic frequency of TLR4 Asp 299 Gly, Thr 399IIl
and TNF- a
(-308G/A) in association with preterm birth. The prevalence of genotype
TLR4 Asp 299 Gly, Thr 399IIl did not differ significantly in
cases and controls. Increased risk of preterm birth was found with AA
genotype of TNF-a
(OR 1.5; CI 1.02, 2.20; P=0.03; i.e. AA Vs GA+GG).
TLR4 mRNA expression (Fig 1) was found to be higher (P<0.001)
in cases [0.7 (0.04)] than in controls [0.5 (0.04)].
TABLE I Details of the Neonates and Mothers Enrolled
|
Characteristics |
Cases |
Controls |
|
(n=559) |
(n=559) |
|
Neonatal details |
|
|
|
Birthweight (kg)* |
1985 (473.7) |
2768.6 (423.9) |
|
Gestational age (wks)* |
33.9 (1.9) |
38.2 (1.0) |
|
Gestational age (wks)# |
34 (27-36) |
38(37-42) |
|
Gestation £34 wk, n (%) |
119 (21.2%) |
0 |
|
Male sex, n (%) |
318 (56.9) |
314 (56.2) |
|
Head Circumference (cm)* |
30.9 (1.7) |
33 (1.3) |
|
Length (cm)* |
43.2 (3.0) |
46.6 (3.7) |
|
Maternal details |
|
|
|
Age (y)* |
25.8 (4.2) |
25.7 (3.7) |
|
Weight (kg)*‡ |
52.9 (7.4) |
56.3 (6.8) |
|
Gravida <3, n (%) |
390 (69.7%) |
420 (75.1%) |
|
Parity <3, n (%) |
424 (75.8%) |
443 (79.2%) |
|
*mean (SD); #Median (Range);‡P<0.001. |
|
|
TABLE II Genotypic Frequency of TLR4 Asp 299Gly, Thr 399 Ile and TNF-a Polymorphisms
|
Gene |
Cases, n (%) |
Controls, n (%) |
P value |
|
AA |
495 (88.5%) |
497 (89%) |
0.81 |
|
AG |
64 (11.9%) |
61 (11%) |
|
|
CC |
515 (92%) |
531 (95%) |
0.053 |
|
CT |
44 (8%) |
28 (9%) |
|
|
GG |
307 (55%) |
318 (57%) |
|
|
GA |
180 (32%) |
190 (34%) |
0.2 |
|
AA |
72 (13%) |
51 (9%) |
0.1 |
|
Dominant Model |
|
|
|
|
GG |
307 (55%) |
318 (57%) |
0.5 |
|
GA+AA |
252 (45%) |
241 (43%) |
|
|
Overdominant Model |
|
|
|
|
GA |
180 (32%) |
191 (34%) |
0.5 |
|
GG+AA |
379 (68%) |
368 (66%) |
|
|
Recessive Model |
|
|
|
|
AA |
72 (13%) |
50 (9%) |
0.03 |
|
GG+GA |
487 (87%) |
509 (91%) |
|
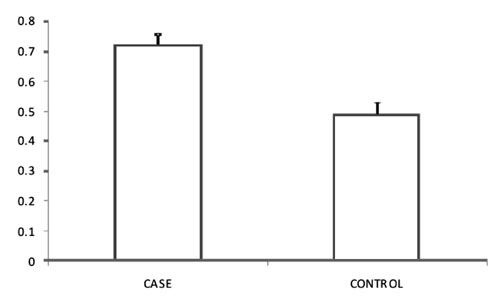 |
|
Fig.1 Quantitative Real time-PCR
analysis of TLR-4 gene expression which showed 2.5 fold increase
in cases as compared to controls; the y axis of 2(– DCt)
represents the relative gene expression of TLR-4.
|
Discussion
In this study, increased expression of TLR4 mRNA
levels was reported in cases as compared with controls. However, no
association was found between TLR4 SNP rs4986790 (A+896 G) and rs4986791
(C+1196T) and TNF-a
SNP rs361525 with preterm birth.
Our findings are supported by results from another
study [4] that reported increased expression of TLR4 in patients with
preterm labour [4]. Our study also concurs with the findings of few
other studies [5-8] which observed TLR4 as a contributing factor in
inflammation. Patni, et al. [9] reported no difference of TLR 4
expression in term and preterm placenta.
A study conducted in Netherland by Krediet, et al.
[10] found no association of TLR4 (Thr 399 IIl) polymorphism with
the gestational age. While Lorenz, et al. [11] reported that
TLR4 T allele was higher in singleton preterm neonates as compared
to multiple preterm neonates and term neonates. Elovitz, et al.
[12] reported that TLR4 G allele (Asp 299 Gly) populates
are more at risk of premature rupture of membrane before or at 33 weeks.
In case of TNF- a
(-308G/A), no association was found in our
study; similar results are seen in few earlier studies [13-15]. A study
conducted on maternal-fetal genotype interface showed that mother
carrying TNF-a-308GA
genotype and fetus with the carrier of TNF-a-308GG
genotype are at risk of preterm birth [16]. Speer, et al. [17]
also supported the higher incidence of inflammation associated preterm
birth in fetus with TNF-a-308GG genotype.
We conclude that the increased level of TLR4 in
preterm birth supports its role in causation of preterm birth.
Contributors: SA: planned the study; prepared the
manuscript; contributed to patient enrolment. MP: collected and analyzed
the data. Both authors contributed to manuscript writing, and its final
approval.
Funding: Indian Council of Medical Research;
Competing interest: None stated.
|
What This Study Adds?
•
Increased TLR4 mRNA levels in
mother may be associated with the risk of preterm birth.
|
References
1. Pandey M, Awasthi S. Prognostic role of
interluekin-1 a
and b gene
polymorphisms in preterm birth. Gene Rep. 2016;4:112-7.
2. Pandey M, Chauhan M, Awasthi S. Interplay of
cytokines in preterm birth. Ind J Med Res. 2017;146:316-27.
3. Pfaffl MW. A new mathematical model for relative
quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45.
4. Pawelczyk E, Nowicki BJ, Izban MG, Pratap S,
Sashti NA, Sanderson M, et al. Spontaneous preterm labor is
associated with an increase in the proinflammatory signal transducer
TLR4 receptor on maternal blood monocytes. BMC Pregnancy Childbirth.
2010;10:66.
5. Robertson SA, Wahid H, Chin P, Hutchinson M,
Moldenhauer L, Keelan J. Toll-like Receptor-4: A New Target for Preterm
Labour Pharmacotherapies? Curr Pharm Des. 2018;24:960-73.
6. Li L, Kang J, Lei W. Role of Toll-like receptor 4
in inflammation-induced preterm delivery. Mol Hum Reprod. 2010;16:267-72.
7. Noguchi T, Sado T, Naruse K, Shigetomi H, Onogi
A, Haruta S, Kawaguchi R, et al. Evidence for activation of
Toll-like receptor and receptor for advanced glycation end products in
preterm birth. Mediators Inflamm. 2010:2010:490-506.
8. Chin YP, Dorian CL, Hutchinson MR, Olson DM,
Rice KC, Moldenhauer LM, et al. Novel Toll-like receptor-4
antagonist (+)-naloxone protects mice from inflammation-induced preterm
birth. Sci Rep. 2016:6:1-13.
9. Patni S , Aled H. Bryant A , Louise P. Wynen b ,
Anna L, et al. Functional activity but not gene expression of
toll-like receptors is decreased in the preterm versus term human
placenta. Placenta. 2015:36:1031-8.
10. Krediet TG, Wiertsema SP, Vossers MJ , Hoeks SB ,
Fleer A, Ruven HJ, et al. Toll-likereceptor 2 polymorphism is
associated with preterm birth. Pediatr Res. 2007;62:474-6.
11. Lorenz E,
Hallman M, Marttila R, Haataja R, Schwartz DA. Association between the
Asp299Gly polymor-phisms in the Toll-like receptor 4 and premature
births in the Finnish population. Pediatr Res. 2002;52:373-6.
12. Elovitz MA, Wang Z, Chien EK, Rychlik DF,
Phillippe M. A new model for inflammation-induced preterm birth: the
role of platelet-activating factor and Toll-like receptor-4. Am J Pathol.
2003;163:2103-11.
13. Kalinka J, Bitner A. Selected cytokine gene
polymorphisms and the risk of preterm delivery in the population of
Polish women. Ginekol Pol. 2009;80:111-7.
14. Jafarzadeh L, Danesh A, Sadeghi M, Heybati F,
Hashemzadeh M. Analysis of relationship between tumor necrosis factor
alpha gene (G308A Polymorphism) with preterm labor. Int J Prev Med.
2013;4:896-901.
15. Andalas M, Hakimi M, Nurdiati DS, Astuti I, Imran
I, Harapan H. Association of 308G/A TNF- a
gene polymorphism and spontaneous preterm birth in Acehnese Ethnic
Group, Indonesia: This polymorphism is not associated with preterm
birth. Egypt J Med Hum Genet. 2016;17:33-40.
16. Yilmaz Y, Verdi H, Taneri A, Yazici AC, Ecevit
AN, Karakas NM, et al. Maternal fetal proinflammatory cytokine
gene polymorphism and preterm birth. DNA Cell Biol. 2012;31:92-7.
17. Speer EM, Gentile DA, Zeevi A, Pillage G, Huo D,
Skoner DP. Role of single nucleotide polymorphisms of cytokine genes in
spontaneous preterm delivery. Hum Immunol. 2006;67:915-23.
|
|
|
 |
|

