|
|
|
Indian Pediatr 2018;55: 211-215 |
 |
Bronchopulmonary
Dysplasia in Preterm Neonates in a Level III Neonatal Unit in
India
|
|
Savita Bhunwal 1,
Kanya Mukhopadhyay1,
Shalmoli Bhattacharya2,
Pranab Dey3 and
Lakhbir Kaur Dhaliwal4
From Departments of 1Pediatrics, 2Biophysics,
3Cytology, and 4Obstetrics and Gynecology,
Postgraduate Institute of Medical Education and Research, Chandigarh,
India.
Correspondence to: Dr Kanya Mukhopadhyay, Professor,
Neonatology, Department of Pediatrics, PGIMER, Chandigarh 160 012,
India.
Email: [email protected]
Received: January 02, 2017;
Initial review: April 11,2017;
Accepted: November 24, 2017.
Published online:
December 14, 2017.
PII:S097475591600101
|
|
Objective: To find out the
incidence and associations of bronchopulmonary dysplasia (BPD) in
preterm neonates.
Design: Descriptive cohort.
Methods: All consecutively born
neonates <33 weeks gestation requiring oxygen or respiratory support
during first 3 days of life were enrolled from a level III neonatal unit
in Chandigarh, India. Those with malformations were excluded. Placenta
was examined for histological chorioamnionitis in preterm rupture of
membranes and/or preterm spontaneous onset of labour. Serum
Malondialdehyde (MDA) and Superoxide dismutase (SOD) and Catalase levels
were estimated on day 3 of life. All recruited neonates were followed up
till discharge or death.
Results: Out of 250 neonates
enrolled, 170 (68%) survived till day 28 and BPD developed in 19 (11.2%)
infants. The mean gestation and birth weight were significantly lower in
infants who developed BPD. Chorioamnionitis (clinical 5.3% vs
1.9%, P=0.375; and histological 37.5% vs 16.7%, P<0.001),
patent ductus arteriosus (PDA) (52.6% vs 8.9%, P<0.001),
median (IQR) sepsis episodes [2 (2,3) vs 1 (1,2), P<0.001],
invasive ventilation (84.2% vs 11.3%, P<0.001), and
duration of ventilation [56 (4) d vs 4 (5) d, P=0.001]
were significantly higher in infants with BPD. Serum MDA, SOD and
Catalase levels were comparable between the two groups.
Conclusion: Chorioamnionitis,
PDA and sepsis were significantly associated with BPD.
Keywords: Anti-oxidant enzymes,
Chorioamnionitis, Chronic lung disease, Oxidants.
|
|
B
roncho-pulmonary dysplasia (BPD) is one of the
most important chronic complications in very preterm neonates [1],
especially those born before 28-30 weeks of gestation and weighing
£1000 grams
[2]. Ehrenkranz, et al. [3] validated the consensus definition of
BPD in a cohort of preterm (<32 weeks) extremely low birth weight (ELBW)
infants alive at 36 weeks of post menstrual age (PMA) and reported an
incidence of 44%, as against the older criteria [3]. BPD decreases with
increasing gestational age and birth weight with the highest incidence
at lower extremes of birth weight and gestation age [3,4].
In India, with the increasing availability of
surfactant and intensive care, survival at lower gestational age is
steadily increasing; however, data regarding the incidence and risk
factors of BPD are scarce [5,6]. This study was planned to generate
recent data on incidence and risk factors of BPD in infants <33 weeks of
gestation.
Methods
This study was carried out at a level III neonatal
unit in Chandigarh, India between July 2012 and June 2013 after
clearance by Institutional research ethics committee. All consecutively
born infants <33 weeks gestational age, who received any form of
respiratory support (oxygen by hood/nasal cannula or continuous positive
airway pressure (CPAP) or non-invasive mechanical ventilation (NIMV) or
mechanical ventilation (MV) within first 3 days of life were
prospectively enrolled. Infants with congenital malformations (including
congenital heart disease other than PDA) were excluded. Written informed
consent was obtained from parents. Gestational age was based on maternal
last menstrual period and postnatally by New Ballard Score [7]. Neonates
were followed up till discharge or death during hospital stay.
BPD was defined based on the criterion of receiving
oxygen therapy of >21% for 28 days in those who received initial
respiratory support. Severity of BPD was assessed at 36 weeks PCA. Mild
BPD was defined as a need for supplemental oxygen (O2) for
³28 days but not at
36 weeks postmenstrual age (PMA) or discharge, moderate BPD as need for
£30% O2 at 36
weeks PMA and severe BPD as need for
³30% O2 (CPAP, HFNC,
and/or positive pressure) at 36 weeks PMA [3]. No baby was discharged on
home oxygen.
In cases of preterm rupture of membrane (PTROM)
and/or preterm spontaneous onset of labor at <33 weeks of gestation,
where infants developed respiratory distress soon after birth, placenta
was examined by a histopathologist to look for any evidence of
Chorio-amnionitis. Clinical chorioamnionitis was diagnosed in the
setting of maternal fever ( ³100.4ºF/38º
C) and at least two of the following: maternal leukocytosis (>15,000
cells/mm3), maternal
tachycardia (>100 bpm), fetal tachycardia (>160 bpm), uterine
tenderness, stained or foul smelling amniotic fluid. Cases of maternal
upper respiratory infection and urinary tract infection were excluded
[11]. Histological chorioa-mnionitis was diagnosed in presence of
amniotropic infiltration by both maternal and fetal neutrophils in the
chorioamniotic membranes and the umbilical cord [12]. Pneumonia
was diagnosed if there was respiratory distress, in the presence of a
positive blood culture or if any two of the following were present -
existing or predisposing factors like maternal fever, foul smelling
liquor, prolonged rupture of membranes or gastric polymorphs more than 5
per high power field, Clinical picture of septicemia (poor feeding,
lethargy, poor reflexes, hypo- or hyper-thermia, abdominal distension
etc.), X-ray suggestive of pneumonia, and positive septic screen.
Sepsis was defined as either blood culture positive sepsis, or
suspect sepsis if septic screen was positive in presence of clinical
features but blood culture negative. All antenatal details including
clinical chorioa-mnionitis, demographic characteristics and morbidities
including details of ventilation were recorded.
Serum Malondialdehyde (MDA) and antioxidant enzymes
(Superoxide dismutase (SOD) and Catalase) were estimated on day 3 of
life in all infants. MDA level was measured as described by Ohkawa
(1979), et al. [8], SOD was measured by method reported by Kono,
et al. [9], and Catalase was estimated by method described Luck,
et al. [10].
Each year in our institution, nearly 600-700 infants
are born at <33 weeks gestation, of which approximately 50% receive
respiratory support, with average survival of about 63% [6]. Hence it
was expected 250-300 infants could be enrolled in this study with about
160-190 infants surviving.
Statistical analysis was performed using SPSS version
18.0. Comparisons of demographic character-istics and co-morbidities
were made using Student’s t test or chi square test, as
appropriate in two groups (BPD vs No BPD).
Results
A total of 670 infants were born at <33 weeks of
gestation, of which 350 infants required respiratory support within the
first 72 hours of life (Fig. 1). Demographic details of
the study population is described in Table I.
TABLE I Demographic and Morbidity Characteristics of the Study Participants (N=250)
|
Characteristic |
Value |
|
#Gestational age (wk) |
29 (2.1) |
|
Gestational age categories |
|
<28 wk |
41 (16.6) |
|
28 to 30 wk |
111 (44.4) |
|
31 to 32 wk |
98 (39.4) |
|
#Birthweight (g) |
1203 (335) |
|
Birthweight categories |
|
<1 kg |
74 (29.6) |
|
1-1.5 kg |
124 (49.6) |
|
>1.5 kg |
52 (20.8) |
|
SGA |
82 (32.8) |
|
Male gender |
147 (58.8) |
|
Mechanical Ventilation |
33 (13.2) |
|
Only CPAP |
110 (44) |
|
Suspect sepsis |
203 (81.2) |
|
Culture positive sepsis |
31 (12.4) |
|
PDA (ECHO-proven) |
29/224 (12.9) |
|
ROP (n=106) |
61 (57.5) |
|
Values expressed as n (%) or #Mean(SD). |
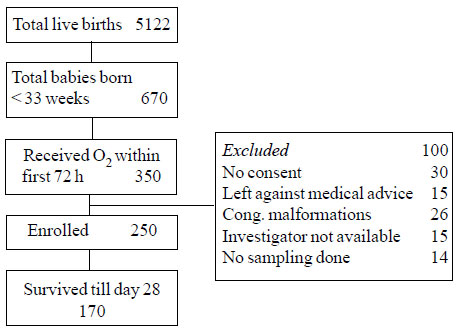 |
|
Fig. 1 Consort flow diagram for the
study.
|
Of the 250 infants enrolled, 170 (68%) survived till
day 28 of life and 19 (11.2%) developed BPD. Severity of BPD could be
assessed in 16 infants as one baby died and two infants were off oxygen
at 36 weeks PCA. Amongst all infants <33 weeks gestation born during the
study period (n=670) who survived till 28 days, BPD incidence was
3.9%.
Infants with BPD were lesser in gestation, lighter at
birth, more likely to be growth retarded, and had a higher occurrence of
chorioamnionitis and PTROM (Table II). When stratified by
gestation, BPD was more frequent in infants <28 weeks (7 out of 16) than
those between 28-30 weeks (10 out of 70). Similarly, infants weighing <1
kg had a higher incidence of BPD than those between 1-1.5 kg (8 out of
34 vs 9 out of 91).
TABLE II Demographic and Clinical Characteristics of Infants With and Without Bronchopulmonary Dysplasia
|
Variable |
BPD, n=19 |
No BPD, n=151 |
P value |
RR/RD (95% CI) |
|
#Birth weight (g) |
1090 (312) |
1305 (304) |
0.004 |
- 215(-34 , -378) |
|
#Gestation (wk) |
9 (47.4%) |
35 (23.2%) |
0.023 |
2.58(1.12, 5.93) |
|
Antenatal steroid |
11 (57.9%) |
80 (52.98%) |
0.686 |
1.19(0.51, 2.82) |
|
Clinical chorioamnionitis |
1 (5.3%) |
3 (1.99%) |
0.375 |
2.31(0.40, 13.3) |
|
Histological chorioamnionitis |
6/16 (37.5%) |
10/60 (16.7%) |
<0.001 |
5.06(1.63, 15.76) |
|
PTROM |
12 (63.2%) |
51(33.8%) |
<0.001 |
2.91(1.21, 7.01) |
|
Values are expressed as n(%) or #mean(SD); BPD:
bronchopulmonary dysplasia; SGA: Small for gestational age,
PTROM: Preterm prolonged rupture of membrane. |
Infants with BPD had a significantly higher
prevalence of PDA, pneumonia, sepsis, need for blood transfusions, and
need for invasive ventilation (Table III).
TABLE III Comparison of Co Morbidities In Infants With or Without Bronchopulmonary Dysplasia
|
Variables |
BPD (n=19) |
No BPD (n=151) |
P value |
|
HMD |
11 (57.9) |
75 (49.7) |
0.499 |
|
Surfactant |
14 (73.6) |
59 (39.1) |
0.004 |
|
PDA |
10 (52.6) |
11 (7.3) |
<0.001 |
|
Pneumonia |
4 (21) |
10 (6.6) |
0.031 |
|
#Sepsis episodes |
2 (2-3) |
1 (1-2) |
<0.001 |
|
Sepsis (All) |
19 (100) |
116 (76.8) |
<0.001 |
|
Culture-positive sepsis |
11 (57.9) |
36 (23.8) |
0.002 |
|
Blood transfusion |
16 (84) |
22 (14.5) |
<0.001 |
|
CPAP within 48 h (n=144) |
18 (94.7) |
126 (83.4) |
0.197 |
|
Invasive ventilation at 48 h |
8 (42.1) |
9 (6) |
<0.001 |
|
Mechanical ventilation |
16 (84.2) |
17 (11.3) |
<0.001 |
|
#Duration of ventilation (d) |
48 (37-60) |
2 (1-6) |
<0.001 |
|
ROP (All stages) |
19/19 (100) |
42/87 (48.3) |
<0.001 |
|
#Hospital stay (d) |
56 (50-76) |
22 (14-35) |
<0.001 |
|
All values in n(%), except #Median (IQR); BPD –
Bronchopulmonary dysplasia; HMD – Hyaline membrane disease; PDA
– Patent Ductus Arteriosus; CPAP – Continuous Positive Airway
Pressure; ROP – Retinopathy of Prematurity. |
There was no difference in the levels of MDA and
antioxidant enzymes (Catalase and SOD) in those with and without BPD (Fig.
2).
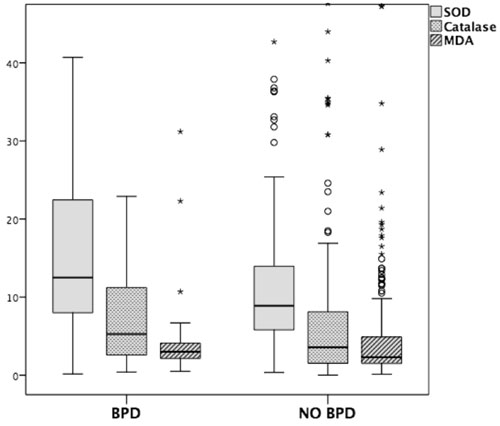 |
|
Fig. 2 Box and Whisker plot showing
Superoxide dismutase (SOD), Catalase and Malondialdehyde (MDA)
levels in infants with or without bronchopulmonary dysplasia
(BPD) on day 3 of life.
|
Discussion
In the present study 11.2% preterm neonates (<33 wk
gestation) with respiratory distress developed BPD with a higher
incidence in infants <1 kg and <28 weeks gestation. PTROM, histological
chorioamnionitis, pneumonia, sepsis, mechanical ventilation, PDA were
present in higher proportion in BPD infants along with longer duration
of ventilation.
The limitations of our study are small sample size
due to time constraints, and lower rates of survival at extremes of
gestation due to infrastructure limitations.
There has been an increase in incidence of BPD (from
5.8% to 11.5%) in the past decade in our institution [5,6]. Narang,
et al. [5] reported an incidence of BPD of 28.7%, 10.7% and 1.7% in
infants less than 28 weeks, 29-30 weeks and 31- 32 weeks, respectively,
and 50%, 8.1% and 2.3% in infants with birthweight <1000 g, 1000-1249 g
and 1250-1499 g, respectively [5]. Ehrenkranz, et al. [3]
reported BPD incidence of 52% in infants with birthweight 501 to 750 g,
34% in infants weighing 750-1000 g, and 15% in infants weighing between
1001-1200 g while it was 7% in infants weighing 1201-1500 grams. We
observed a much lower incidence of BPD at corresponding birthweights
probably due to higher gestation as well as lower rate of survival at
lower gestation. Higher proportion of BPD in SGA infants as compared to
AGA infants can probably be explained by significantly lower gestational
age of SGA infants in our cohort which was also observed in our previous
study [6]. The overall gestational age of our BPD infants is higher
(mean 28.3 weeks) than those reported in Western literature [3]. BPD
developing at higher gestation in our set-up may be due to higher
incidence of sepsis and related factors rather than prematurity alone as
compared to Western data. Reports of BPD from developing countries are
infrequent. Ho and Chang [13] from Malaysia reported an incidence of
3.3% BPD in their VLBW cohort of 2003 infants (mean gestation 29.5 wk),
of whom 72% infants were ventilated. A recent report of Malaysian
national neonatal registry reported a similar incidence of BPD (8-11.7%)
in their VLBW cohort [14], as in the present study.
Gagliardi, et al. [15] reported 15.9%
incidence of BPD in a cohort of 23-32 weeks and <1500 grams infants and
observed mechanical ventilation, greater severity of illness as measured
by Clinical Risk Index for Infants (CRIB) score, and PDA to be
significantly associated risk factors for BPD. Use of antenatal steroids
had no independent effect on BPD. These findings are similar to the
findings of the present study. There are conflicting reports of use of
continuous positive airway pressure (CPAP) and reduction of BPD. A
Cochrane systematic review showed no difference in BPD defined as oxygen
dependency in preterm neonates [16]. Boo, et al. [14] noted that
CPAP significantly reduced BPD among survivors in a cohort of VLBW
infants in Malaysia. Narang, et al. [5] also reported higher
incidence of BPD in ventilated infants and who also received higher
oxygen concentration. PTROM and histological chorioamnionitis was
detected more amongst BPD infants in the present study. A recent large
cohort study did not find any association of BPD and chorioamnionitis
[17]. Sepsis and pneumonia were significantly higher in infants with
CLD, as also reported in a previous study [5].
Though free radicals play a role in BPD pathogenesis,
the present study did not find elevated levels of free radicals or
deficiency of anti-oxidant enzymes in the BPD infants. These are similar
to the observations of Ryan, et al. [18], where only pulmonary
concentrations of free radical product malondialdehyde was noted to be
elevated but this elevation was weakly correlated with the development
of BPD. The probable explanation may be that these oxidant biomarkers
are elevated in other conditions like sepsis or pneumonia and the
oxidative injury is only one amongst the multiple factors that plays a
role in development of BPD.
With improving neonatal survival in our country, we
may experience more and more children with BPD. Recognition of
country-specific risk factors like sepsis, pneumonia, PDA,
chorioamnionitis may help us to reduce the incidence of BPD. Adequate
infrastructure will be required for optimum long-term management of
these infants.
Contributors: SB: prepared the protocol, enrolled
patients, collected and analyzed the data and drafted the manuscript;
KM: conceptualized and designed the study, supervised data collection
and analysis, and critically revised the manuscript; SB: conducted
biochemical analysis and reviewed the manuscript. PD: Conducted the
histopathological examination of placenta. LKD: Helped in data
collection and reviewed the manuscript.
Funding: None. Competing interests: None
stated.
| |
|
What is Already Known?
•
Extreme prematurity, PDA, prolonged ventilation, and
invasive ventilation are common risk factors for BPD.
What This Study Adds?
•
BPD occurs in higher gestational age and birthweight infants
in India; chorioamnionitis, sepsis and pneumonia are commonly
associated.
|
References
1. Fanaroff AA, Wright LL, Stevenson DK, Shankaran S,
Donovan EF, Ehrenkranz RA, et al. Very low birth weight outcomes
of the National Institute of Child Health and Human Development Neonatal
Research Network, May 1991 through December 1992. Am J Obstet Gynecol.
1995;173:1423-31.
2. Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA,
Stoll BJ, et al. Very low birth weight outcomes of the National
Institute of Child Health and Human Development Neonatal Research
Network, January 1995 through December 1996. NICHD Neonatal Research
Network. Pediatrics. 2001;107:E1.
3. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright
LL, Fanaroff AA, et al. National Institutes of Child Health and
Human Development Neonatal Research Network. Validation of the National
Institutes of Health consensus definition of bronchopulmonary dysplasia.
Pediatrics. 2005;116:1353-60.
4. Kinsella J, Greenough A, Abman SA.
Bronchopulmonary dysplasia. Lancet. 2006;367:1421-31.
5. Narang A, Kumar P, Kumar R. Chronic lung disease
in neonates: emerging problem in India. Indian Pediatr. 2002;39:158-62.
6. Mukhopadhyay K, Louis D, Murki S, Mahajan R, Dogra
MR, Kumar P. Survival and morbidity among two cohorts of extremely low
birth weight neonates from a tertiary hospital in northern India. Indian
Pediatr. 2013;50: 1047-50.
7. Ballard JL, Khoury JC, Wedig K, Wang L,
Eilers-Walsman BL, Lipp R. New Ballard Score expanded to include
extremely premature infants. J Pediatr. 1991;119:417-23.
8. Ohkawa H, Ohishi N, Yagi K. Assay for lipid
peroxides in animal tissues by thiobarbituric acid reaction. Ann Biochem.
1979;95:351-8.
9. Kono Y. Generation of superoxide radical during
autoxidation of hydroxylamine and an assay for superoxide dismutase.
Arch Biochem Biophys. 1978;186:189-95.
10. Luck H. Catalase, method of enzymatic analysis.
In: Bermeyer HO, editor. London; New York: Academic Press. 1971. p.
855-93.
11. Newton ER. Chorioamnionitis and intraamniotic
infection. Clin Obstet Gynecol. 1993;36:795-808.
12. Redline RW, Faye-Petersen O, Heller D, Qureshi F,
Savell V, Vogler C. Amniotic infection syndrome: nosology and
reproducibility of placental reaction patterns. Pediatr Dev Pathol.
2003;6:435-48.
13. Ho JJ, Chang AS. Changes in the process of care
and outcome over a 10 year period in a neonatal nursery in a developing
country. J Trop Pediatr. 2007;53:232-7.
14. Boo NY, Cheah IG, Neoh SH, Chee SC. Malaysian
National Neonatal Registry. Impact and challenges of early continuous
positive airway pressure therapy for very low birth weight neonates in a
developing country. Neonatology. 2016;110:116-24.
15. Gagliardi L, Bellù R, Rusconi F, Merazzi D, Mosca
F. Antenatal steroids and risk of bronchopulmonary dysplasia: a lack of
effect or a case of over-adjustment. Paediatr Perinat Epidemiol.
2007;21:347-53.
16. Ho JJ, Subramaniam P, Davis PG. Continuous
distending pressure for respiratory distress in preterm infants.
Cochrane Database Syst Rev. 2015;4:CD002271.
17. Ballard AR, Mallett LH, Pruszynski JE, JB Cantey
JB. Chorioamnionitis and subsequent bronchopulmonary dysplasia in
very-low-birth weight infants: a 25-year cohort. J Perinatol.
2016;36:1045-8.
18. Ryan R, Ahmed Q, Lakshminrusimha S. Inflammatory
mediators in the immunology of bronchopulmonary dysplasia. Clinic Rev
Allergy Immunol. 2008;34: 174-90.
|
|
|
 |
|

