|
|
|
Indian Pediatr 2018;55:206-210 |
 |
Effect of Gastric
Lavage on Meconium Aspiration Syndrome and Feed Intolerance in
Vigorous Infants Born with Meconium Stained Amniotic Fluid –
A Randomized Control Trial
|
|
Shrishail Gidaganti, MMA Faridi, Manish Narang and
Prerna Batra
From Division of Neonatology, Department of
Pediatrics, University College of Medical Sciences and Guru Teg Bahadur
Hospital, Delhi, India.
Correspondence to: Dr MMA Faridi, Flat # G-4, Plot #
14, Block-B, Vivek Vihar Delhi 110 095, India.
Email: [email protected]
Received: April 17, 2017;
Initial review: June 20, 2017;
Accepted: January 03, 2018.
Trial Registration: CTRI/2014/03/004495
|
Objective: To compare the incidence of meconium aspiration syndrome
and feed intolerance in infants born through meconium stained amniotic
fluid with or without gastric lavage performed at birth.
Setting: Neonatal unit of a teaching hospital in
New Delhi, India.
Design: Parallel group unmasked randomized
controlled trial.
Participants: 700 vigorous infants of gestational
age ³34
weeks from through meconium stained amniotic fluid.
Intervention: Gastric lavage in the labor room
with normal saline at 10 mL per kg body weight (n=350) or no
gastric lavage (n=350). Meconiumcrit was measured and expressed
as £30%
and >30%.
Outcome Measures: Meconium aspiration syndrome,
feed intolerance and procedure-related complications during 72 h of
observation.
Results: 5 (1.4%) infants in lavage group and 8
(2.2%) in no lavage group developed meconium aspiration syndrome (RR
0.63, 95% CI 0.21, 1.89). Feed intolerance was observed in 37 (10.5%)
and 53 infants (15.1%) in lavage and no lavage groups, respectively (RR
0.70, 95% CI 0.47, 1.03). None of the infants in either group developed
apnea, bradycardia or cyanosis during the procedure.
Conclusion: Gastric lavage performed in the labor
room does not seem to reduce either meconium aspiration syndrome or feed
intolerance in vigorous infants born through meconium stained amniotic
fluid.
Keywords: Neonate, Prevention, Respiratory distress, Risk
factors, Vomiting.
|
|
Meconium stained amniotic fluid (MSAF) complicates 7%
to 22% deliveries, with meconium aspiration syndrome (MAS) developing in
approximately 10% of babies with MSAF [1,2]. Amount of meconium in the
amniotic fluid, fetal acidemia and fetal heart rate are some of the
factors determining the risk of MAS [3,4]. The infant born through MSAF
may ingest or aspirate meconium in utero, during delivery or
after birth. Due to the chemical nature and vasoconstriction action of
the meconium, the MSAF might cause meconium induced gastritis leading to
feed intolerance. After birth, infant may also vomit and aspirate MSAF
resulting in ‘secondary meconium aspiration syndrome’ [5]. Gastric
lavage soon after birth is advocated to prevent these complications.
Some clinicians advise gastric lavage with normal saline but others
advocate use of soda bicarbonate for stomach wash [6-8]. Interestingly,
none of the practices of gastric lavage is based on scientific evidence
and is followed at most centers by convention.
The insertion of infant feeding tube in the stomach
is an invasive procedure and might cause immediate complications like
apnea, bradycardia, cyanosis and traumatic injury and late adverse
effects like impaired sensitivity to pain [6-9]. We, therefore, planned
this study to compare the frequency of MAS and feed intolerance between
the infants born through MSAF with and without gastric lavage performed
in the labor room, and also to evaluate safety of the procedure.
Methods
This parallel group unmasked randomized controlled
trial was conducted in the Division of Neonatology of University College
of Medical Sciences and GTB Hospital, Delhi, India. The study was
approved by the institutional Ethics Committee for Human Research.
Written informed consent was obtained from the mother before delivery.
Assuming proportion of MAS as 15% among those born
with MSAF [10], level of significance 5%, power 80%, and 50% relative
difference of MAS in infants with or without gastric lavage, a sample
size of 700 infants (350 in each group) was calculated to be sufficient.
Study participants included neonates of both genders with gestational
age ³34 weeks,
who were born through MSAF and were vigorous at birth. Babies with gross
congenital anomalies, those born to mothers with suspected
chorioamnionitis, and those receiving methyldopa during pregnancy were
excluded from the study. The gestational age was calculated by Naegle’s
rule and Modified Ballard’s scoring [11]; latter was taken into account
if difference in the gestational age estimated by two methods was
greater than two completed weeks.
Eligible newborns were randomized through computer
generated random numbers to assign them into intervention (lavage) or
control group. Coding scheme was concealed in serially numbered, opaque,
and sealed envelopes by a person not directly involved in the study.
Parents were informed of the group, their infant was included in, after
randomization.
About 15 mL MSAF was obtained in a clean kidney tray
with the help of a sterilized plain rubber catheter No.10 passed through
vagina or directly in the kidney tray during delivery. In cases of
caesarean section, liquor was collected in a 20 mL disposable syringe by
an obstetrician after giving uterine incision. Meconiumcrit was assessed
by centrifuging 10 mL MSAF at 1000 rpm for 10 min in a 20 mL glass test
tube.
The infant was placed under radiant warmer. Oxygen
sensor of the Pulse Oximeter (Welch Allyn, USA) was attached to the
ulnar aspect of the right wrist. After initial stabilization in the
labor room, gastric lavage was carried out in the intervention group. An
orogastric tube (#10 Fr) was passed into the stomach, after measuring
the length as per the standard procedure, and lavage was done with
normal saline (10 mL/kg body weight). In control group, gastric lavage
was not performed. The heart rate, oxygen saturation, apnea and color of
the infant were monitored during gastric lavage and till 20 min after
removal of orogastric tube. In control group also, above parameters were
recorded for same the duration. Heart rate <120 bpm and >160 bpm were
taken as bradycardia and tachycardia respectively. Apnea was defined as
cessation of breathing for >20 sec or for any duration associated with
cyanosis or bradycardia. SpO 2
< 85% after 15 min of birth was taken as significantly low. Local trauma
to the oropharynx was assessed by naked eye examination twice in first
12 h of life. Breastfeeding was started within 60 min after normal
delivery and within two hours after caesarean section. X-ray
chest was obtained within 4 h in all infants, and was repeated if infant
developed respiratory distress.
The respiratory distress was monitored by Downe’s
score at birth [13] and repeated every 6 h for first 24 h, and every 12
h for next 48 h. When Downe’s score was
³3 a repeat X-ray
chest was obtained and MAS was treated as per standard treatment
protocol. MAS was defined as the presence of respiratory distress in an
infant born through MSAF, whose symptoms could not be otherwise
explained and with radiological evidence of meconium aspiration [14].
Intolerance to enteral feeding was evaluated one hour after initiation
of breastfeeding and every six hour thereafter for 72 h. Feed
intolerance was considered if there was history of vomiting at least two
times in 24 h, or there was pre-feed aspirate of >50% of previous feed
even once in 24 h in case baby was fed expressed breast milk by
orogastric tube, or increase in abdominal girth by 2 cm on two occasions
in 24 h. Vomiting was differentiated from regurgitation by the
associated features like retching/ tachycardia/ salivation/ sweating
[15]. When a baby developed feed intolerance, gastric lavage was carried
out with normal saline if it was not done earlier; weight and urine
output were monitored and breastfeeding was continued as per the unit
protocol. In case infant continued with feed intolerance after 24 h, a
repeat gastric lavage was done with the normal saline and breastfeeding
was continued.
Primary outcome measure was proportion of infants
developing meconium aspiration syndrome within 72 h of age in both
groups. Secondary outcome measures were proportion of infants developing
feed intolerance after initiation of breastfeeding till 72 h in two
groups and number of babies showing adverse effects of gastric lavage
(apnea, bradycardia, cyanosis, local trauma).
Statistical analysis: Data were analyzed by SPSS
16.0 statistical software. Meconiumcrit was compared in two groups by
Student’s ‘t’ test. The comparison of the qualitative variables such as
MAS and feed intolerance in the study and control groups were done using
Chi square test. P value of <0.05 was considered as statistically
significant.
Results
A total of 700 infants (350 each in intervention and
control group) were enrolled and successfully completed the study (Fig.
1). The baseline demographic and clinical characteristics of
enrolled infants are presented in Table I.
TABLE I Comparison of Perinatal and Neonatal Parameters in Study and Control Groups
|
Variables |
Intervention |
Control group |
|
group (n=350) |
(n=350) |
|
Perinatal Characteristics |
|
Primipara |
190 (54.3) |
185 (52.9) |
|
Meconiumcrit |
|
|
|
≤30% |
249 (71.1) |
260 (74.3) |
|
>30% |
101 (28.9) |
90 (25.7) |
|
Fetal bradycardia |
46 (13.1) |
41 (11.7) |
|
Fetal tachycardia |
16 (4.6) |
14 (4.0) |
|
Caesarian delivery |
129 (36.9) |
109 (31.1) |
|
Forceps/ Vacuum |
5 (1.4) |
6 (1.7) |
|
Breech |
8 (2.3) |
3 (0.9) |
|
APGAR Score* |
|
1 min |
8.9 (0.3) |
8.9 (0.3) |
|
5 min |
9.0 (0.3) |
9 (0.3) |
|
Neonatal Characteristics |
|
Male gender |
182 (52.0) |
193 (55.1) |
|
Preterm birth |
94 (26.9) |
97 (27.4) |
|
Birth weight (g)* |
2684 (398) |
2706 (398) |
|
Length (cm)* |
48.1 (1.4) |
48.2 (1.4) |
|
Head circumference (cm)* |
33.5 (0.8) |
33.6 (0.8) |
|
Values in No. (%) or *mean (SD). |
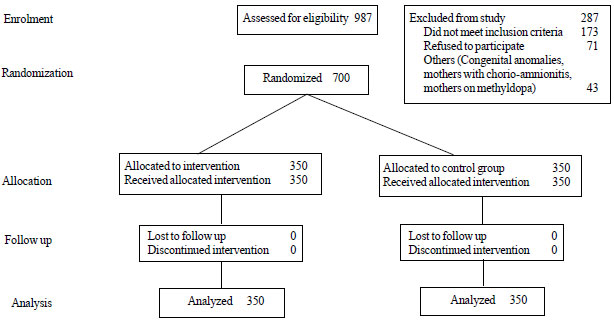 |
|
Fig. 1 Consort flow diagram for the
study.
|
A significant difference was noted in number of
vomiting episodes in first 24 hours (P=0.001), while no
statistical difference was noted in incidence of MAS and feed
intolerance in the two groups (Table II). Gastric lavage
prevented occurrence of first episode of vomiting within 6 hour of
initiation of breastfeeding but did not affect eventual development of
feed intolerance. Overall, feed intolerance developed in 90 (12.8%)
infants after initiation of breastfeeding. However, all infants improved
after 48 h of age (Table III). Out of 90 infants who
suffered from feed intolerance, 63 (70%) were born with meconiumcrit
£30%, and in
27 (30%) babies, the meconiumcrit was >30% (P>0.05).
TABLE II Effect of Gastric Lavage on Development of Meconium Aspiration Syndrome and Feed Intolerance
|
Outcome |
Intervention group (n=350), No.(%) |
Control group (n=350), No.(%) |
RR (95% CI) |
|
Meconium aspiration syndrome |
5 (1.4) |
8 (2.2) |
0.63 (0.21, 1.89) |
|
Feed Intolerance |
37 (10.5) |
53 (15.1) |
0.70 (0.47, 1.03) |
|
Vomiting episodes in 24 h |
76 |
115 |
0.66 (0.52, 0.85) |
|
0-6 h |
30 (8.5) |
47 (13.4) |
0.64 (0.41, 0.96) |
|
6-12 h |
32 (9.1) |
38 (10.8) |
0.84 (0.54, 1.32) |
|
12-24 h |
14 (4) |
30 (8.5) |
0.47 (0.25, 0.86) |
|
Vomiting episodes in 24-48 h |
6 (1.7) |
10 (2.8) |
0.38 (0.15, 0.95) |
No baby developed apnea, bradycardia or local trauma
in the study group; SpO2 <
85% at 15 min was observed in one baby in the control group and two
babies in the intervention group (P>0.05).
Discussion
In this randomized controlled trial on vigorous
infants born through meconium stained amniotic fluid, we documented that
gastric lavage performed immediately after birth in labor room did not
reduce the incidence of MAS and feed intolerance. No procedure related
complication was observed following gastric lavage.
Investigators could not be blinded for intervention
due to the nature of intervention, and results cannot be generalized on
non-vigorous infants, constitute few study limitations. Also, the study
was not adequately powered to detect smaller changes in the incidence of
MAS and feeding intolerance.
In a similar study from India, Sharma, et al.
[6] randomized 267 babies in gastric lavage and 269 babies to no gastric
lavage group. They followed up infants for development of retching,
vomiting and secondary meconium aspiration syndrome till the time they
were discharged from the hospital. None of the babies developed
secondary meconium aspiration syndrome in any group [6]. Evidence from
several other studies also does not support gastric lavage preventing
feed intolerance in infants born with MSAF [5,7,15-17]. A recent
systematic review by Deshmukh, et al. [18] concluded that gastric
lavage may improve feed tolerance in neonates born to MSAF; however
small sample size in included studies, and probable bias were the
limitations. Our study is in conformity with above observations that
routine gastric lavage in MSAF babies does not seem to prevent
development of MAS, irrespective of the concentration of meconium in the
amniotic fluid, mode of delivery or birthweight. Gastric lavage also
does not seem to reduce incidence of feed intolerance either, though the
first episode of vomiting after initiation of breastfeeding may be
prevented. However, the procedure of gastric lavage appears safe,
without immediate complications like apnea, bradycardia, cyanosis or
local trauma. We recommend further studies addressing the issue of MAS
and feed intolerance on non-vigorous infants to generate stronger
evidence in favor or against gastric lavage performed in the labor room.
Contributors: SG: data collection and
prepared initial draft of manuscript; MMAF: conceptualized study,
analyzed and scrutinized data, and finalized the manuscript; MN:
supervised data collection and contributed to manuscript writing; PB:
reviewed literature, manuscript editing and analysis. All authors
approved final version of the manuscript.
Funding: None; Competing interest: None
stated.
|
What is Already Known?
•
Gastric lavage in infants born through meconium stained
amniotic fluid is performed to prevent secondary meconium
aspiration syndrome and feed intolerance.
What This Study Adds?
•
Gastric lavage in
vigorous infants born through meconium stained amniotic fluid
does not seem to reduce the incidence of meconium aspiration
syndrome or feed intolerance.
|
References
1. Bhat R, Rao A. Meconium-stained amniotic fluid and
meconium aspiration syndrome: a prospective study. Ann Trop Paediatr.
2008;28:199-203.
2. Narang A, Nari PMC, Bhakoo ON. Management of
meconium stained amniotic fluid: A team approach. Indian Pediatr.
1989;30:9-13.
3. Trimmer KJ, Gilstrap LC 3rd. "Meconiumcrit" and
birth asphyxia. Am J Obstet Gynecol. 1991;165:1010-3.
4. Rossi EM, Philipson EH, Williams TG, Kalhan SC.
Meconium aspiration syndrome: intrapartum and neonatal attributes. Am J
Obstet Gynecol. 1989; 161:1106-10.
5. Sharma P, Nangia S, Tiwari S, Goel A, Singla B,
Saili A. Gastric lavage for prevention of feeding problems in neonates
with meconium stained amniotic fluid : A randomized controlled trial.
Peditar Int Child Health. 2014;34:115-9.
6. Narachi H, Kulayat N. Is gastric lavage needed in
neonates with meconium-stained amniotic fluid? Eur J Pediatr.
1999;158:315-7.
7. Ameta G, Upadhyay A, Gothwal S. Role of gastric
lavage in vigorous neonates born with meconium stained amniotic fluid.
Indian J Pediatr. 2013;80:195-8.
8. Anand KJS, Runeson B, Jacobson B. Gastric suction
at birth associated with long term risk for functional intestinal
disorders in later life. J Pediatr. 2004;144:449-54.
9. Stoll B. The Newborn Infant. In: Kliegman
R, Jenson H, Behrman R, Stanton B. editors, Nelson’s Textbook of
Pediatrics (18thed). Saunders Elsevier Publications India. 2008. p.
675-83.
10. Bahl D. A Study of Neonatal Outcome in Relation
to Meconiumcrit of Liquor Amnii. MD Thesis, University of Delhi, 1993.
11. Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman
BL, Lipp R. New Ballard Score, expanded to include extremely premature
infants. J Pediatr. 1991; 119:417-23.
12. Bhat S. Respiratory distress in newborns In:
Achar’s textbook of Pediatrics, 4th ed. Universities Press; 2009. p.
221.
13. Wiswell TE, Bent RC. Meconium staining and the
meconium aspiration syndrome: Unresolved issues. Pediatr Clin N Am.
1993;50:955-81.
14. JeevaSankar M, Agarwal R, Mishra S, Deorari AK,
Paul VK. Feeding of low birth weight infants. Neonatology protocols.
Indian J Pediatr. 2008;75:459-69.
15. Shah L, Shah GS, Singh RR, Pokharel H, Mishra OP.
Status of gastric lavage in neonates born with meconium stained amniotic
fluid: A randomized controlled trial. Italian J Pediatr. 2015;41:85.
16. Garg J, Masand R, Tomar BS. Utility of gastric
lavage in vigorous neonates delivered with meconium stained liquor: A
randomized controlled trial. Int J Pediatr. 2014;2014:204807.
17. Cuello-García C, González-López V, Soto-González
A.Gastric lavage in healthy term newborns: A randomized
controlled trial. Ann Pediatr (Barc). 2005;63:509-13.
18. Deshmukh M, Balasubramanian H, Rao S, Patole S.
Effect of gastric lavage on feeding in neonates born through meconium-stained
liquor: A systematic review. Arch Dis Child Fetal Neonatal Ed.
2015;100:F394-9.
|
|
|
 |
|

