|
|
|
Indian Pediatr 2017;54: 211-214 |
 |
Endotracheal Aspirate
Microscopy, Cultures and Endotracheal Tube Tip Cultures for
Early Prediction of Ventilator Associated Pneumonia in Neonates
|
|
Mahendra Kumar Gupta, Jayashree Mondkar, *Anjali
Swami, Deepraj Hegde and Sorabh Goel
From the Departments of Neonatology and
*Microbiology, Lokmanya Tilak Municipal Medical College and General
Hospital, Sion. Mumbai, India.
Correspondence to: Dr Mahendra Kumar Gupta,
Department of Neonatology, Lokmanya Tilak Municipal Medical College and
General Hospital, Sion, Mumbai 400 022, India.
Email: [email protected]
Received: January 31, 2016;
Initial review: June 06, 2016;
Accepted: December 27,2016.
Published online: February 02, 2017.
PII:S097475591600036
|
Objective: To evaluate the utility of
endotracheal aspirate microscopy, culture and endotracheal tube tip
culture for early diagnosis of ventilator-associated pneumonia in
neonates. Methods: Inborn ventilated neonates were
followed-up for ventilator-associated pneumonia using Center for Disease
Control and Prevention (CDC) criteria. Endotracheal aspirate microscopy,
culture and endotracheal tube tip cultures were performed. Results:
Ventilator-associated pneumonia occurred in 28/68 (41%) neonates as
per CDC criteria. Endotracheal aspirate microscopy ( ≥5
polymorphonuclear cells per high power field) and endotracheal aspirate
culture had 78.6% and 75% sensitivity, 87.5% and 90% specificity,
positive predictive value of 81.5% and 84%, and negative predictive
value of 85.4% and 83.72%, respectively. Mean (SD) time of result of
microscopy and endotracheal aspirate culture was 55.7 (4.3) h and 108.3
(19.7) h, respectively in comparison to diagnosis made at 143.5 (23.3)
h, as per CDC criteria. Conclusion: Endotracheal aspirate
microscopic examination and culture can be supportive in objective
diagnosis of ventilator-associated pneumonia with an added advantage of
earlier prediction.
Key words: Complications, Diagnosis, Intensive care,
Nosocomial sepsis.
|
|
V
entilator support is essential part of care in
neonatal intensive care unit (NICU), but ventilator-associated pneumonia
(VAP) can occur as a serious complication. National Nosocomial
Infections Surveillance system (NNIS) data (2004) from USA reported
pooled mean VAP rate of 1.4-3.5/1000 ventilator days [1]. In developing
countries, the reported rates of VAP are significantly higher, ranging
from 16.1 to 89 episodes per 1,000 ventilator days [2,3].
Center for Disease Control and Prevention (CDC)
criteria for diagnosis of VAP includes multiple parameters which are
observer dependent, and does not include cultures, which are important
for appropriate antibiotic therapy [4]. Patients with inadequate
antibiotic therapy may have a poor prognosis if a change in regimen is
delayed while awaiting microbiological results [5]. Bronchoalveolar
lavage (BAL) and protected specimen brush (PSB) have been reported to
have high sensitivity and specificity for the diagnosis of VAP [6,7],
but are invasive and difficult to perform in neonates due to small
endotracheal tube (ET) size. Endotracheal aspirate (ETA) is relatively
noninvasive method that can be easily performed through small ET in
NICU. We aimed to study the utility of ETA microscopy and cultures for
diagnosis of VAP.
Methods
This study was conducted in a tertiary care NICU over
a period of one year (April 2014 to March 2015). All inborn infants
admitted in the NICU requiring invasive ventilation for more than 48
hour were included after obtaining parental consent. Infants with
suspected or diagnosed congenital pneumonia, critical congenital cardiac
disease, life-threatening congenital and chromosomal anomalies or
pulmonary haemorrhage were excluded.
Infants who required ventilatory support were
intubated by oro-tracheal route in NICU or labour room, and then put on
Neonatal Ventilators (Maquet servo-i, Avea standard or Schiller graph
NET advance). Disposable ventilator circuits (RT 126 dual limb infant
ventilator circuits, Fisher and Paykel) were connected on ventilator
through servo-controlled humidifier (Fisher and Paykel MR850). The
disposable tubings were not changed till extubation or until visible
soiling. Humidifier was filled with sterile water by a closed system.
All ventilated babies were nursed in the supine position, and routine
care as per NICU protocol was provided. Suctioning and collection of
sample for ETA was done by an open suction using Hagedorn method [10],
when first suction was required for the presence of secretions.
Suctioning was performed by using sterile feeding tube or suction
catheter and syringe. If the yield was <1 mL, the procedure was repeated
by instilling 0.5 mL of normal saline (drawn from a freshly opened
ampoule) into the endotracheal tube. Sample collected in the syringe was
sent for quantitative culture and microscopic analysis along with sample
of saline taken for suction. Studied infants were carefully followed up
for signs of VAP. This included, apart from clinical examination,
regular recording of body temperature, ventilator setting (peak
inspiratory pressure, positive end expiratory pressure and fractional
oxygen (FiO 2)), arterial
blood gas, leukocyte count, serial C-reactive protein, blood culture and
chest radiographs. Blood culture was done as a part of the sepsis
work-up profile. No infant received steroid, local or oral antibiotic, H2
blocker or proton pump inhibitor.
Mean (SD) intubation-to-collection time of ETA sample
was 53.2 h (3.9) which was same for ET aspirate microscopy and ET
aspirate culture. ET tip sample was taken either at 1st ET
replacement or during extubation whichever was earlier. ET tube tip (1
cm) was cut by sterile surgical blade and taken into a culture tube
directly and was sent for culture at mean (SD) 115.2 (40.8) hrs. The
diagnosis of VAP was made on the CDC criteria for infants less than or
equal to 1 year of age [4].
A smear was prepared from ETA for Gram staining to
determine polymorphonuclear (PMNL) cells and the type of organism.
Microscopy was done at high magnification (x400) by using VISION 2000
LED microscope and recorded as: PMNL count: >10/HPF; 5-10/HPF or <5/HPF
or Nil /HPF. Smear of ETA was scored as per Bartlett scoring system [11]
and bacteria were classified as gram positive or negative; cocci or
bacilli. Culture of ETA and ET Tip was done on Blood and Mac Conkey
agar. Antibiotic sensitivity was done on Muller Hinton agar and results
were expressed as colony forming units/mL. With quantitative analysis of
ETA and ET tube tip, the threshold for diagnosing VAP in this study was
considered as 10 5CFU/mL
[12].
Data analysis was done by calculating mean and
standard deviation for continuous data and using unpaired t test/ Mann
whitney U test for statistical significance. Pearsonís chi-square test
or fisherís exact test was used for categorical data. P value <0.05 was
considered as statistically significant. Statistical analysis was done
using the software SPSS version 17.0 for windows
Results
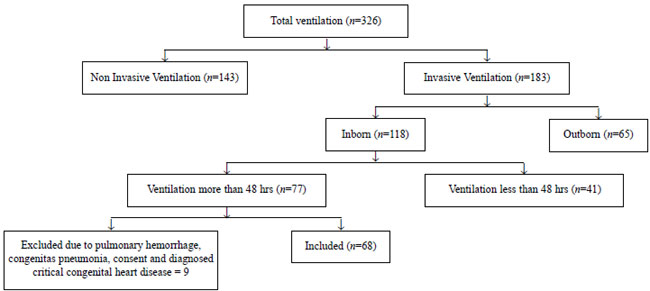
A total of 326 neonates were screened out of which 68
were included in the study (Fig. 1). The baseline
demographic data of the study population were comparable (Table
I). Twenty-eight (41.2%) neonates developed VAP. Mean (SD) time of
ET aspirate sample collection was 53.2 (2.9) h for (SD) microscopy and
culture, and of ET tube tip sample was 115.2 (40.8) h. The mean (SD)
time of result of endotracheal aspirate microscopy and endotracheal
aspirate culture was 55.7 (4.3) h and 108.3 (19.7) h, respectively in
comparison to diagnosis made at 143.5 (23.3) h as per CDC criteria (P<0.001).
 |
|
Fig.1 Flow of patients in the study.
|
TABLE I Baseline Demographic and Risk Factors in the Study Population
|
Variable |
VAP (28) |
NO VAP (40) |
|
Age (wks) * |
36.9 (1.8) |
38 (1.7) |
|
Weight (kg) *
|
2.3 (0.6) |
2.7 (0.5) |
|
Male gender |
15 (53.6%) |
21 (52.5%) |
|
Meconium stained amniotic fluid |
17 (60.7%) |
15 (37.5%) |
|
Vaginal delivery |
22 (78.6%) |
30 (75%) |
|
Resuscitation at birth
|
|
Bag and mask ventilation |
7 (25.0%) |
8 (20%) |
|
Intubation |
13 (46%) |
6 (15%) |
|
APGAR at 5 minutes <7 |
19 (67.9%) |
11 (27.5%) |
|
No. of endotracheal tube changes*
|
1.82 (0.67) |
1.65 (0.66) |
|
Duration of ventilation*
|
14.07 (2.23) |
10.18 (2.02) |
|
Mortality (all cause) |
9 (32.1%) |
10 (25%) |
|
*values in mean (SD); VAP: Ventilator-associated pneumonia. |
The sensitivity, specificity, negative predictive
value and positive predictive value of ETA microscopy ( ≥5
PMNL/HPF and >10 PMNL/HPF), ETA culture (colony count >105
CFU/mL), endotracheal tube tip culture (colony count >105
CFU/mL) are shown in Table II. In our study, organisms
cultured in ETA were Acinetobactor (29.4%), Klebsella (4.4%),
Pseudomonas (3%), MRSA (3%); mixed growth was seen in one case.
TABLE II Results of Microscopy and Culture
|
Variable |
VAP (n=28) |
No VAP (n=40) |
Sensitivity (%) |
Specificity(%) |
PPV (%) |
NPV (%) |
|
ETA microscopy ≥5 PMNL/HPF
|
22 (78.6%) |
5 (12.5%) |
78.6 |
87.5 |
81.5 |
85.4 |
|
ETA microscopy >10 PMNL/HPF
|
13 (46.4%) |
4 (10%) |
46.4 |
90.0 |
76.5 |
70.6 |
|
ET aspirate culture colony count >105 cfu/mL |
21 (75%) |
4 (10%) |
75.0 |
90.0 |
84.0 |
83.72 |
|
ET tip culture colony count >105 cfu/mL |
11 (39.3%) |
3 (7.5%) |
39.3 |
92.5 |
78.6 |
68.5 |
|
Gram Staining organism |
13 (46.4%) |
2 (5%) |
46.4 |
95 |
86.7 |
71.7 |
|
VAP- ventilator associated pneumonia; PPV-positive
predictive value; NPV-negative predictive value; ETA-endotracheal
tube aspirate; PMNL-polymorphonuclear cells. |
Discussion
In this study, ETA microscopy (≥
5PMNL/HPF) and ETA culture colony count >105
CFU/mL were found to be useful for early diagnosis
of VAP with significantly lower diagnosis time. No significant
complications of ETA were found in this study.
The limitations of our study were it being a single
center study with a small sample size. Our trial had enrolment of mainly
near term and full term infants, and the findings may not be
generalizable to the preterm population. Also, we did not repeat ETA for
documenting normalization.
The ETA culture sensitivity and specificity when cut
off was >10 5 CFU/mL for
diagnosis of VAP in our study was comparable to findings noted by
Labenne, et al. [6] where samples were retrieved by BAL
technique. The mean time to diagnosis of VAP as per CDC criteria in our
study was comparable to that documented by Tripathi, et al. [3],
but detection of VAP could be done earlier by us using ETA microscopy
and culture.
We conclude that ETA culture colony count (>10 5
CFU/mL) and ETA microscopy ≥5PMNL/HPF
is supportive in the objective diagnosis of VAP with added advantage of
early diagnosis.
Contributors: MKG and DH conceptualized and
designed the study, analyzed data and drafted the manuscript; AS:
collected the data and helped in data analysis; JM: supervised patient
management; SG: literature search and helped in data analysis. All
authors approved the final manuscript.
Funding: none; Competing interest: None
stated.
|
What This Study Adds?
∑ Endotracheal aspirate microscopic
examination and culture can be supportive to CDC criteria in
objective diagnosis of VAP with an added advantage of earlier
prediction.
|
References
1. National Nosocomial Infections Surveillance System
Report: Data summary. Am J Infect Control. 2004;32:470-85.
2. Foglia E, Meier M, El-ward A.
Ventilator-associated pneumonia in neonatal and pediatric intensive care
units. Clin Microbiol Rev. 2007;20:409-25.
3. Tripathi S, Malik GK, Jain A. Study of
ventilator associated pneumonia in neonatal intensive care unit:
characteristics, risk factors and outcome. Internet J Med Update.
2010;5:12-9.
4. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes
JM. CDC definitions for nosocomial infections, 1988. Am J Infect
Control. 1988;16:128-40.
5. Luna CM, Vujacich P, Niederman M. Impact of BAL
data on the therapy and outcome of ventilator-associated pneumonia.
Chest. 1997;111:676-85.
6. Labenne M, Poyart C, Rambaud C, Goldfarb B, Pron
B, Jouvet P, et al. Blind protected specimen brush and
bronchoalveolar lavage in ventilated children. Crit Care
Med. 1999;27:2537-43.
7. Gauvin F, Dassa C, Chaibou M, Proulx F, Farrell
CA, Lacroix J. Ventilator-associated pneumonia in intubated children:
comparison of different diagnostic methods. Pediatr Crit Care
Med. 2003;4:437-43.
8. Hagedorn MI, Gardner SL, Abman SH: Respiratory
diseases; Handbook of Neonatal Intensive Care. St. Louis: Mosby 1989.
381.
9. Bartlett JG, Ryan KJ, Smith TF. Laboratory
Diagnosis of Lower Respiratory Tract Infection. In: Washington JA ed,
Cumitech 7A, Washington DC: American Society for Microbiology.1987. p.
1-18.
10. Marquette CH, Georges H, Wallet F, Ramon P,
Saulnier F, Neviere R, et al. Diagnostic efficiency of
endotracheal aspirates with quantitative bacteria cultures in intubated
patients with suspected pneumonia. Comparison with the protected
specimen brush. Am Rev Respir Dis. 1993;148:138-44.
|
|
|
 |
|

