|
|
|
Indian Pediatr 2014;51:
179-183 |
 |
Neonatal Hearing Screening – Experience from
a Tertiary Care Hospital in Southern India
|
|
Ann Mary Augustine, *Atanu Kumar Jana, *Kurien Anil Kuruvilla, *Sumita
Danda, Anjali Lepcha, Jareen Ebenezer, Roshna Rose Paul, Amit Tyagi and
Achamma Balraj
From the Departments of ENT, *Neonatology and
#Medical Genetics, Christian Medical College, Vellore, TN, India.
Correspondence to: Dr Achamma Balraj, Department of
ENT, Christian Medical College, Vellore,
Tamil Nadu 632 004, India.
Email: [email protected]
Received: June 01, 2013;
Initial review: June 26, 2013;
Accepted: September 20, 2013.
Published online: 2013, October 5.
PII: S097475591300554
|
Objective: To implement a neonatal hearing screening program using
automated auditory brainstem response audiometry in a tertiary care
set-up and assess the prevalence of neonatal hearing loss.
Design: Descriptive study.
Setting: Tertiary care hospital in Southern
India.
Participants: 9448 babies born in the hospital
over a period of 11 months.
Intervention: The neonates were subjected to a
two stage sequential screening using the BERAphone. Neonates suspected
of hearing loss underwent confirmatory testing using auditory steady
state response audiometry. In addition, serological testing for TORCH
infections, and connexin 26 gene was done.
Main outcome measures: Feasibility of the
screening program, prevalence of neonatal hearing loss and risk factors
found in association with neonatal hearing loss.
Results: 164 babies were identified as suspected
for hearing loss, but of which, only 58 visited the audiovestibular
clinic. Among 45 babies who had confirmatory testing, 39 were confirmed
to have hearing loss and were rehabilitated appropriately. 30 babies had
one or more risk factors; 6 had evidence of TORCH infection and 1 had
connexin 26 gene mutation.
Conclusion: Neonatal hearing screening using
BERAphone is a feasible service. The estimated prevalence of confirmed
hearing loss was comparable to that in literature. Overcoming the large
numbers of loss to follow-up proves to be a challenge in the
implementation of such a program.
Keywords: BERA phone, Neonate, Screening, Outcome.
|
|
N
eonatal hearing loss has a prevalence that is
more than twice that of other newborn disorders amenable to screening
such as congenital hypothyroidism and phenyl-ketonuria [1,2].
Congenital, bilateral hearing impairment occurs in approximately 1 to 5
per 1000 live births and when permanent unilateral hearing loss is
included, the incidence increases to 8 per 1000 live births [3-5].
Studies done in India using different hearing screening protocols have
estimated the prevalence of neonatal hearing loss to vary between 1 and
8 per 1000 babies screened [6-8]. Early identification and intervention
for hearing loss by 6 months of age provides better prognosis in
language development, academic success, social integration and
successful participation in the society [5].
The effectiveness and need for univeral hearing
screening in neonates has previously been well proven [9,10]. Although
hearing screening programs using different screening protocols have been
set up in some centres, procedures for systematic identification and
rehabilitation on a large scale are yet to be tested and implemented in
the Indian setting.
Tests used for screening newborns for hearing loss
include Otoacoustic emissions (OAE) and automated Auditory Brainstem
Response audiometry (aABR). While OAE is cheap, quick, simple and
reliable with a sensitivity of 100% and specificity of 99 % [11-13],
aABR has the additional advantage of identifying neonates with auditory
neuropathy unlike testing for OAE. The other advantages of aABR include
rapidity, easy-to-use and high sensitivity (0.99) and specificity (0.87)
[14,15]. The Maico MB11 BERAphone is an aABR system employing a special
headphone [16]. It consists of a hand-held headphone unit which
incorporates a set of three fixed reusable electrodes. It has been
tested and found to have a sensitivity of 99.9% and specificity of 97.9%
when used in a two-stage screening protocol which is comparable to that
of OAE. The test is also seldom affected by ambient noise making it
suitable for use in the postnatal ward [17].
This study was undertaken with the primary objective
of exploring the feasibility of setting up a universal neonatal hearing
screening program in a tertiary care hospital (handling an average of 10
000 deliveries/year), using the BERAphone (Two-stage sequential
screening protocol). The secondary objectives included estimating the
prevalence of neonatal hearing loss in a tertiary care setting, and
assessing the associated risk factors in those identified with hearing
loss.
Methods
This descriptive study was conducted between January
and November of 2010 at our tertiary care center after institutional
research and ethical committee clearance was obtained. Four graduates in
biological sciences were trained for the study, and their knowledge,
ability to obtain informed consent, counsel parents and perform the
screening test was assessed formally at the end of the training period.
The BERAphone consists of a handheld headphone unit
which is positioned on the babies head after application of electrode
gel at the points of contact with the electrodes (vertex and mastoid).
An optimized chirp stimulus is used at 35dB and the system automatically
detects the presence of an auditory brainstem response based on an
implemented statistical test algorithm. If response is detected the test
produces a ‘Pass’ result while failure to detect a response within 180
seconds produces a ‘Refer’ result.
All normal newborn babies delivered in our hospital
were screened by the trained technicians using BERAphone between 24
hours and 72 hours after birth. Newborns admitted in the neonatal
intensive care unit (NICU) were screened prior to discharge from the
NICU (once their general condition was stable). Mothers of all babies
born in the tertiary care hospital were counseled regarding the benefits
of hearing screening, procedure of the screening test, need for
follow-up and further tests if the neonate failed the screening test,
and the interventions available if hearing loss was confirmed. The first
screening test was done in the postnatal wards or NICU after obtaining
informed consent from the mother. Parents of babies who failed (‘refer’)
the screening test were counseled and asked to return after 1 week for
second screening. These babies underwent a second testing in a quiet
room. Those who passed on the second screening were discharged from the
study while those who failed a second time were referred for further
evaluation in the audiovestibular clinic (AVC) at the same centre, where
a detailed history for risk factors [10] was obtained, the babies were
examined, parents were counseled and diagnostic testing using Auditory
Steady State Response Audiometry (ASSR) was done. Repeated phone calls
and letters were used to contact parents of babies who failed to return
for follow-up.
ASSR was used as the diagnostic procedure to confirm
hearing loss, as well as to obtain frequency specific thresholds to
enable more effective and appropriate hearing aid fitting. Distortion
product otoacoustic emission (DPOAE) testing was used in addition, to
detect those with auditory neuropathy. Those confirmed with hearing loss
were followed up in the AVC for further evaluation and appropriate
rehabilitation.
The babies who were referred after screening twice
with BERA phone and whose parents consented for blood tests also
underwent serological tests for known infective causes of hearing loss (Toxoplasma,
Rubella, Cytomegalovirus and Herpes simplex virus) and genetic testing
for the connexin 26 gene mutation. Data obtained was analysed
using SPSS. Rates, ratios and proportions were calculated.
Results
Among 9671 neonates born between 1st January and 30th
November 2010, 9448 (97.7%) were screened for hearing deficit. 223
babies could not be screened since they were critically ill in the
nursery and later died or were discharged at request.
A total of 863 babies were referred on first
screening which implies a discharge rate of 90.9% with single screening.
713 (82.6%) came for second screening and 164 of them were referred
again. The discharge rate after the 2-stage sequential screening with
BERAphone was 98.2% (Fig.1). Of the 9448 babies screened,
2339 were NICU graduates (Table I).
TABLE I Screening Results of Normal Babies and NICU Graduates
|
Babies
|
Babies refered |
Babies who unde- |
Babies refered |
Babies who under- |
Babies with
|
|
screened |
on 1st screening |
went 2nd screening |
on 2nd screening |
went confirmatory |
confirmed
|
|
|
|
|
|
tests |
hearing loss
|
|
Normal |
7109 |
713 |
563 |
150 |
32 |
31 |
|
NICU graduates |
2339 |
150 |
150 |
14 |
14 |
8 |
|
Total |
9448 |
863 |
713 |
164 |
46 |
39 |
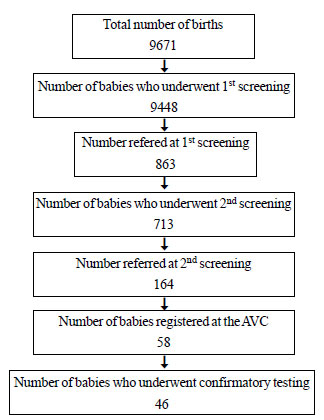 |
|
Fig. 1 Number of babies at each stage
of the screening program.
|
Among 164 babies referred to the AVC, only 58 (35.4%)
registered in the clinic. The remaining 106 babies failed to come for
follow-up despite repeated attempts (phone calls and letters) to contact
the families. Eleven of these children were lost to further follow up
and did not come back for confirmatory tests despite repeatedly
contacting them. One child had died and therefore 46 children underwent
confirmatory testing. The ASSR was done between 1 and 3 months of age.
Thirty nine were confirmed to have hearing loss and 7 had bilateral
normal hearing by ASSR (Fig.2).
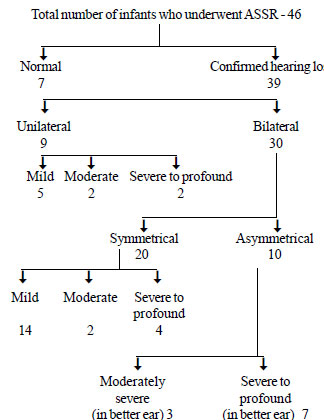 |
|
Fig. 2 Results of ASSR in study
subjects.
|
Table II shows the associated risk factors
[10] identified in the screened babies who had been ‘referred’ after the
second screening. Among 58 neonates, 30 had one or more risk factors.
Three neonates had other congenital anomalies viz. Down’s
syndrome; hydrocephalus, ventricular septal defect and ectopic left
kidney; and microcephaly, thrombocytopenia, hepato-splenomegaly and
patent ductus arteriosus. Parents of 34 neonates consented for blood
tests: screening for TORCH infections and Connexin 26 gene was done. Six
neonates were positive for TORCH infections: 5 were positive for
Cytomegalovirus while one was positive for Rubella. Of these 6 neonates,
3 had severe to profound hearing loss, 2 had mild to moderate hearing
loss and one had normal hearing on ASSR. One neonate out of the 34 was
positive for connexin 26 gene mutation and the ASSR showed severe
to profound hearing loss. The mutation found in this neonate was the
common founder mutation W24X.
TABLE II Associated Risk Factors in 58 Babies Referred Twice on Screening
|
Risk Factor |
No.(%) |
|
Consanguineous marriage |
12(20.7) |
|
Family history of hearing loss |
5(8.6) |
|
H/o in utero infection |
6(10.3) |
|
Family H/o craniofacial anomalies |
2(3.4) |
|
Family H/o syndromes |
3(5.2) |
|
Hyperbilirubinemia (> 20mg/dL) |
3(5.2) |
|
Very low birth weight <1500g |
3(5.2) |
|
Prematurity (gestation <37weeks) |
6(10.3) |
|
H/o Meningitis |
2(3.4) |
|
Low Apgar score (≥4 at 1 min or
≥6 at 5 min)
|
2(3.4) |
|
Mechanical ventilation ( > 5 days) |
1(1.7) |
|
Ototoxic drugs (gentamicin)
|
2(3.4) |
|
Other congenital diseases |
3(5.2) |
All children with confirmed bilateral hearing loss of
moderate degree or more have been fitted with hearing aids and are on
follow-up. Those with severe to profound hearing loss have been advised
cochlear implant. One child has undergone bilateral cochlear implant and
has joined regular school.
Discussion
The selection, training of staff and establishing
procedures for screening were found to be feasible and can be
effectively done in any secondary or tertiary level hospital provided
adequate knowledge about the importance of the program, the procedure
and equipment is available with the supervisory staff. The screening
program required intense supervisory input from the primary investigator
as well as an audiologist. Frequent evaluation of test procedures, entry
of data and supervision of the technicians is required. It is possible,
however, to train a non-ENT surgeon for the supervisory role in the
screening procedure, maintenance of equipment and interpretation of
results.
The BERAphones were quite easy to use and worked very
well in high ambient noise surroundings. Under ideal conditions (sound
proof room and a quiet sleeping child) the BERAphone screening test
takes five minutes to complete. On an average, screening took 10 to 15
minutes to complete in the postnatal ward since the ambient noise in the
ward was more than 50 dB (as recorded in the wards with a sound level
meter). However, the equipment required frequent servicing by the
company and the software required frequent reinstallation. High usage
was the reason attributed. The laptop required constant recharging of
batteries which added to delays and disruption in work and consequently
limited the number of children who could be screened on a given day.
Follow-up (after failing the test the first time) was
intended at 6 weeks after birth. In practice, it was found that the
follow up was poor at 6 weeks and IgM testing for infective causes
required an early sample. Hence the follow up appointment had to be
advanced to one week after discharge. Parents were more likely to come a
week after discharge from the hospital for a checkup hence decreasing
the dropouts. This also had the advantage that those children who failed
the test the second time could be referred for the diagnostic test
earlier.
The waiting time for confirmatory testing was between
1 to 3 months. This was because of the availability of only one testing
facility for both the routine diagnostic testing of patients attending
tertiary care and the neonates identified during the study. Often babies
required multiple attempts to obtain a satisfactory result because of
artifacts produced by upper respiratory tract infections and failure of
the baby to achieve deep sleep. Frequently, patients did not keep
appointments and so had to be rescheduled for another date. Babies with
confirmed hearing loss could be fitted with appropriate hearing aids by
6 months of age and started on auditory verbal therapy thereby
initiating the process of early rehabilitation.
The estimated prevalence of hearing loss among
neonates in this study was 4.1 per 1000 babies screened. Although this
value is similar to that obtained in other studies done in India [6-8],
it is still an underestimation considering the large number of babies
who were lost to follow-up. Nearly 50% of neonates who attended the AVC
after failing the screening test twice had one or more risk factors for
hearing loss. However, babies with risk factors are more likely to be
brought back for follow-up as these children require frequent hospital
visits for various other reasons. The causal association of the
identified risk factors is also difficult to ascertain.
The fact that nearly 98% of the babies born in the
hospital were recruited for the first screening and more than 80% of
those identified on the first screening completed the 2 nd
stage of screening establishes the feasibility of a 2 stage sequential
hearing screening protocol using automated ABR (BERAphone) in a tertiary
care set-up. However, ensuring follow up of children who were referred
twice proved to be the biggest hurdle. Most parents required repeated
counseling and multiple telephone calls to return for confirmatory
tests. In spite of these measures, our study showed a large attrition of
patients. Only 46 of the 164 neonates identified on screening underwent
confirmatory tests. The problem of a huge loss to follow-up is a reality
even in developed countries which have established universal neonatal
hearing screening programs. In the United States, where nearly 95% of
neonates are screened only half of those who do not pass the initial
screening undergo confirmatory testing and rehabilitation [18,19].
Measures to increase awareness regarding neonatal hearing loss, its
effect on the individual and society, available rehabilitation
modalities, and the effectiveness of early identification and
rehabilitation are essential for the successful implementation of such a
program.
We conclude that the BERAphone-based two-step
screening is easy to use effectively by trained technicians for the
implementation of a screening program. The sensitivity and specificity
of the equipment in the test setting however, are to be ascertained. A
large loss to follow-up is the biggest hurdle in the implementation of
such a program.
Acknowledgments: The technicians Ms Ramya, Selvi,
Angel, Indu and Bamini for performing the screening, and Mrs. Revathy
and Thenmozhi for carrying out the ASSR.
Contributors: AMA: drafted the manuscript,
acquisition, analysis and interpretation of data and final approval of
manuscript; AKJ, KAK, SD and AB: concept and design of the study,
critically revising article for important intellectual content and final
approval of manuscript and AL, JE, RRP and AT: acquisition of data,
critically revising article for important intellectual content and final
approval of manuscript.
Funding: Indian Council for Medical Research
(ICMR); Competing interests: None stated.
|
What is Already Known?
•
Universal neonatal hearing
screening has been widely instituted in most developed
countries.
What This Study Adds?
•
The feasibility of a universal neonatal hearing screening
program at a tertiary care set up in a developing country using
automated ABR has been emphasized and the potential hurdles
including a large number of loss to follow-up have been
highlighted.
|
References
1. Fisher DA, Dussault JH, Foley TP, Klein AH,
LaFranchi S, Larsen PR, et al. Screening for congenital
hypothyroidism: results of screening one million North American infants.
J Pediatr. 1979;94:700-5.
2. Bickel H, Bachmann C, Beckers R, Brandt NJ,
Clayton BE, Corrado G, et al. Neonatal mass screening for
metabolic disorders: summary of recent sessions of the committee of
experts to study inborn metabolic diseases. Eur J
Pediatr.1981;137:133–9.
3. Mehra S, Eavey RD, Keamy DG Jr. The epidemiology
of hearing impairment in the United States: newborns, children, and
adolescents. Otolaryngol Head Neck Surg. 2009;140:461-72.
4. Stach BA, RamachandranVS. Hearing disorders in
children. In: Madell JR, Flexer C eds. Pediatric
Audiology: Diagnosis, Technology, and Management. New York: Thieme
Medical Publishers Inc; 2008. P. 3-12.
5. Judith A, Mason MS, Kenneth R, Herrmann MD.
Universal infant hearing screening by automated auditory brainstem
response measurement. Pediatrics. 1998;101:221-8.
6. Nagapoornima P, Ramesh A, Srilakshmi, Rao S,
Patricia PL, Gore M, et al. Universal hearing screening. Indian J
Pediatr. 2007;74:545-9.
7. Paul AK. Early identification of hearing loss and
centralized newborn hearing screening facility- The Cochin experience.
Indian Pediatr. 2011;48:355-9.
8. Rai N, Thakur N. Universal screening of newborns
to detect hearing impairment – Is it necessary?. Int J Pediatr
Otorhinolaryngol. 2013;77:1036-41.
9. Sanders R, Durieux-Smith A, Hyde M, Jacobson J,
Kileny P, Murnane O. Incidence of hearing loss in high risk and
intensive care nursery infants. J Otolaryngol Suppl. 1985;14:28-33.
10. Joint Committee on Infant Hearing. Joint
Committee on Infant Hearing (JICH) 1994 Position Statement. Pediatrics.
1994;95:152-6.
11. De Capua B, De Felice C, Costantini D, Bagnoli F,
Passali D. Newborn hearing screening by transient evoked otoacoustic
emissions: analysis of response as a function of risk factors. Acta
Otorhinolaryngol Ital. 2003;23:16-20.
12. Maxon AB, White KR, Vohr BR, Behrens TR. Using
transient evoked otoacoustic emissions for neonatal hearing screening.Br
J Audiol. 1993;27:149-53.
13. Maxon AB, White KR, Behrens TR, Vohr BR. Referral
rates and cost efficiency in a universal newborn hearing screening
program using transient evoked otoacoustic emissions. J Am Acad Audiol.
1995;6:271-7.
14. Iwasaki S, Hayashi Y, Seki A, Nagura M, Hashimoto
Y, Oshima G, et al. A model of two-stage newborn hearing
screening with automated auditory brainstem response. Int J Pediatr
Otorhinolaryngol. 2003;67:1099-104.
15. van Straaten HL, Hille ET, Kok JH, Verkerk PH.
Dutch NICU Neonatal Hearing Screening Working Group. Implementation of a
nation-wide automated auditory brainstem response hearing screening
program in neonatal intensive care units. Acta Paediatr. 2003;92:332-8.
16. Shehata-Dieler WE, Dieler R, Wenzel G, Keim R,
Singer D, von Deuster Ch. Universal newborn hearing screening program in
Wurzburg. Experience with more than 4000 newborns and the influence of
non-pathological factors on the test results. Laryngorhinootologie.
2002;81:204-10 [German].
17. Cebulla M, Shehata-Dieler W. ABR-based newborn
hearing screening with MB11 BERAphone®using an optimized chirp for
acoustical stimulation. Int J Pediatr Otorhinolaryngol. 2012;76:536-43.
18. Joint Committee on Infant Hearing. Year 2007
Position Statement: Principles and Guidelines for Early Hearing
Detection and Intervention Programs. Pediatrics. 2007;120:898-921.
19. Shulman S, Besculides M, Saltzman A, Ireys H,
White KR, Forsman I. Evaluation of the universal newborn hearing
screening and intervention program. Pediatrics. 2010;126:S19-27.
|
|
|
 |
|

