|
|
|
Indian Pediatr 2013;50: 336-337 |
 |
Hypokalemic Periodic Paralysis and Distal
Renal Tubular Acidosis Associated with Renal Morphological
Changes
|
|
Ratan Gupta, Kumar Saurabh, Shobha Sharma and Riyanka Gupta
From the Department of Pediatrics, Vardhman Mahavir
Medical College and Safdarjung Hospital, New Delhi, India.
Correspondence to: Dr Ratan Gupta, Specialist
Pediatrician at Department of Pediatrics, Vardhman Mahavir Medical
College and Safdarjung Hospital, New Delhi, India.
Email: [email protected]
Received: June 11, 2012;
Initial review: June 26, 2012;
Accepted: October 09, 2012.
|
|
We report an unusual case of 5-yrs-old girl presenting with recurrent
episodic weakness with documented hypokalemia, polyuria and failure to
thrive. The child was finally diagnosed as having distal renal tubular
acidosis. Imaging studies revealed associated hypoechoic spaces in renal
medulla. Long term treatment with alkali and maintenance of normokalemia
lead to regression of these morphological changes.
Key words: Hypoechoeic medullary spaces,
Hypokalemic periodic paralysis, Renal tubular acidosis (RTA).
|
ypokalemic periodic paralysis is a
rare disorder causing recurrent episodic weakness. Most
cases are hereditary due to various channelopathies. Distal
renal tubular acidosis (RTA) is an uncommon secondary cause
of HPP, more so in children, with only few cases reported
till date. We report a case of HPP due to distal RTA who was
also found to have renal medullary hypoechoeic changes.
Case Report
A 5 yr old girl presented with weakness
of all four limbs and neck without any preceding history of
diarrhea or other illness. There was past history of two
similar episodes of weakness in last 2 years, all occurring
in evening and resolved spontaneously after variable period
of time. Parents also gave history of polydipsia, polyuria
and craving for salty foods. On examination, limbs were
hypotonic with absent reflexes and a motor power of grade
one in all limbs. There was no history of dysarthria,
diplopia, respiratory difficulty or bladder and bowel
involvement. Her weight (10 kg) and height (85cm) were below
3 rd percentile
for age. She was born of non-consanguineous marriage and was
third in birth order.
Her serum sodium was 133 mEq/L (135-148),
serum potassium 1.4 mEq/L (3.5-4.5), serum chloride 105 mEq/L
(95-105), with ECG changes of hypokalemia. Intravenous
therapy followed by oral potassium treatement brought serum
potassium to 3.3 mEq/L. Serum calcium profile revealed a
value of 8.4 mg/dL, Phosphorus of 3.1 mg/dL and alkaline
phosphatase of 461 IU/L. Arterial blood gas analysis showed
normal anion gap (14 mEq/L) metabolic acidosis with a pH of
7.28 (7.35-7.45), serum bicarbonate was 14 mEq/L. Blood
sugar, renal and liver function tests were within normal
limits.
Urine output was about 5 mL/kg/hr, with
pH of 7.0, specific gravity of 1.005. Urine electrolytes
showed urinary potassium of 25 meq/L and there was gross
urinary potassium wasting as 24-hrs urinary potassium was
189 mEq/L (normal: 40-80). Fractional excretion of potassium
was 22%. Urinary anion gap was positive (49 mEq/L)
indicating decreased ammonium chloride secretion. Urine
examination did not show any glucose, proteins or pus cells.
Urinary calcium excretion was high as 24-hrs urinary calcium
excretion was 193 mg in 24 hrs (> 4 mg/kg/d). Ammonium
chloride test was carried out by giving 0.1 mg/kg ammonium
chloride orally after obtaining blood pH, serum bicarbonate
and urine pH. Six hours monitoring of these parameters
revealed lack of acidification of urine in spite of
increasing acidosis of blood from 7.40 to 7.25 suggesting
distal RTA. Altogether all the tests were consistent with
features of distal RTA.
X-ray wrist showed frank rickets with
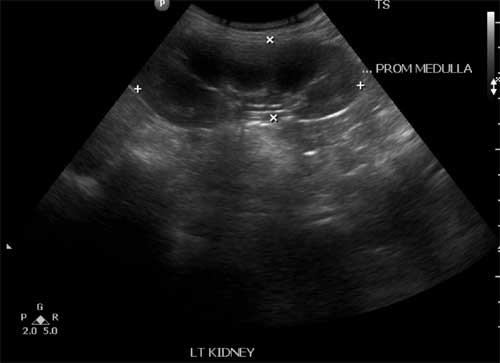
metaphyseal cupping and fraying. USG abdomen showed enlarged
kidney with multiple hypoechoeic spaces in renal medulla of
variable sizes in both kidneys taking shapes of all renal
pyramids but density was not consistent with frank cysts (Fig.
1). Cortcomedullary differentiation was maintained. To
further delineate the structure of kidney CECT abdomen was
done, which showed medullary prominence with relative
thinned out cortex. Child was put on alkali therapy in the
form of soda bicarbonate and polycitra solution along with
calcium supplementation. After two years of therapy child is
now asymptomatic, with serum sodium of 141 mEq/L, potassium
of 3.9 mEq/L, chloride of 103 mEq/L, calcium of 10.5 mg/dL,
bicarbonate of 24 mEq/L and arterial pH of 7.41. 24 hrs
calcium excretion was 48 mg (normal <4 mg/kg/day). Patient
has also achieved her weight and height around median for
his age and sex. Imaging studies revealed a regression of
hypoechoeic medullary spaces with a normal size kidney.
 |
|
Fig.1 USG abdomen showing
multiple hypoechoic spaces of variable sizes in
renal medulla.
|
Discussion
RTA is a recognized cause of severe
hypokalemia and muscle paralysis in adults [1] but there are
only few case reports showing such severe hypokalemia with
distal RTA in children. Chang, et al. [2] reported 3
Chinese girls with HPP secondary to different types of RTA.
In our case, we did not get other possible cause of
secondary distal RTA like liver disorder, drug, toxins,
urological disorders, rhabdomyolysis, which suggests the
possibility of primary (hereditary) or sporadic cause of
distal RTA in this patient, although mutational studies were
not carried out to confirm this assumption. In literature,
distal RTA is associated with renal cysts and medullary
sponge kidney (MSK) which is also regarded to have causal
association with distal RTA [3-5].
In our patient, imaging studies did not
show any renal cyst or MSK, rather we had a very unusual
presentation of hypoehoeic regions taking shape of renal
pyramids in both kidneys and same was confirmed with CECT
abdomen in form of prominent medulla. Ultrasonographic
appearance of neonatal kidney is different from childrens
and adults, the immature cortex in the neonate is thinner
relative to the size of the pyramids so pyramids appear
relatively large and hypoechoeic. Hypoechoeic renal pyramids
may be a normal finding in neonates and infants but not in
childrens [6].
Normally cyst formation is assumed to
occur due to enhanced growth and proliferation of epithelial
cells lining the cysts [7] as it has been seen that
hypokalemia stimulates protein synthesis and cell division
under experimental conditions [8,9]. This shows that severe
hypokalemia promotes formation of renal cysts. In one study
Torres, et al. [10] compared patients with
aldosteronism and hypokalemia to those with controls with
essential hypertension. They conclude that renal cysts
formation may be due to severe hypokalemia rather than a
simple association as removal of adrenal adenomas lead to
regression of cysts which also corresponded to the
normalization of serum potassium levels.
In this case, after two years of
treatment and regular follow up there was regression of
hypoechoeic spaces of renal medulla on imaging studies.
Based on these observations, we postulate that hypoechoeic
renal changes may be the initial steps in cyst formation
which regressed due to early therapeutic intervention.
Furthermore hypokalemia may be the etiological factor for
these changes as long term correction of hypokalemia
resulted in regression of these lesions.
Contributors’: RTG: Diagnosis and
management of case, critical revision, approval; KS:
concept, data analysis, drafting, literature review,
critical revision, approval; SS: critical revision, approval
and RYG: data analysis, literature review, approval.
Funding: None; Competing interests:
None stated.
References
1. Finsterer J. Primary periodic
paralysis. Acta Neurol Scand. 2008;117:145-58.
2. Chang YC, Huang CC, Chiou YY, Yu CY.
Renal tubular acidosis complicated with hypokalemic periodic
paralysis. Pediatr Neurol. 1995;13:52-4.
3. Jayasinghe KS, Mendis BL, Mohideen R,
Ekanayake R, Sheriff MH, Dharmadasa K. Medullary sponge
kidney presenting with hypokalaemic paralysis. Postgrad Med
J. 1984; 60:303-4.
4. Gamakaranage C, Rodrigo C, Jayasinghe
S, Rajapakse S. Hypokalemic paralysis associated with cystic
disease of the kidney. BMC Nephrol. 2011;12:16.
5. Carboni I, Andreucci E, Caruso MR,
Ciccone R, Zuffardo O, Genuardi M, et al. Medullary
sponge kidney associated with Primary distal tubular
acidosis and mutation of H-ATPase genes. Nephrol Dial
Transplant. 2009;24:2734-8.
6. Daneman A, Navarro OM, Somers GR,
Mohanta A, Jarrín JR, Traubici J. Renal pyramids: focused
sonography of normal and pathologic processes. Radiographics.
2010;30:1287-1307.
7. Grantham JJ. Polycystic kidney
disease-an old problem in new context. N Engl J Med. l988;
319:944-6.
8. Walsh MM, Toback FG. Kidney epithelial
cell growth is stimulated by lowering extracellular
potassium concentration. Am J Physiol. 1983;244:429-32.
9. Novak-Hofer 1, Kung W, Eppenberger U.
Role of extracellular electrolytes in the activation of
ribosomal protein S6 kinase by epidermal growth
factor.insulin like growth factor 1 and insulin in ZR-75-1
cells. J Cell Biol. 1988;106:395-401.
10. Torres VE, Young WF Jr, Offord KP, Hattery RR.
Association of hypokalemia, aldosteronism, and renal cysts.
N Engl J Med. 1990;322:345-51.
|
|
|
 |
|

