|
|
|
Indian Pediatr 2013;50:
295-299 |
 |
Locally-Prepared Ready-to-Use Therapeutic
Food for Children with Severe Acute Malnutrition:
A Controlled Trial
|
|
Govind Singh Thakur, HP Singh and Chhavi Patel
From the Department of Pediatrics, Gandhi Memorial
Hospital and SS Medical College, Rewa, MP, India.
Correspondence to: Dr Govindsingh P Thakur,
Kochar ward, Hinganghat, Wardha, Maharashtra, 442301, India.
Email: [email protected]
Received: June 14, 2011;
Initial review: July 06, 2011;
Accepted: August 31, 2012.
Published online: 2012, October 05.
PII: S097475591100501
|
|
Objective: To compare the efficacy of locally-prepared ready-to-use
therapeutic food (LRUTF) and locally-prepared F100 diet in
promoting weight-gain in children with severe acute malnutrition
during rehabilitation phase in hospital.
Study design: Non-randomized
Controlled trial.
Setting: Pediatric ward of
tertiary care public hospital in Central India.
Study period: 1 October, 2009 to
30th May, 2010.
Subjects: Children aged 6 to 60
months, diagnosed as severe acute malnutrition and hospitalized during
study period.
Intervention: Random group
allocation followed for selection of intervention and control cohorts.
The control cohort enrolled during October 1, 2009 to January 31, 2010
received F100 while the intervention cohort enrolled during 1 February
to 15 May 2010 received LRUTF. Subjects receiving either of the two
therapeutic foods were temporally separated to minimize the spillover
effect. The study subjects and the technician delegated for measuring
weight was blinded for type of intervention.
Primary outcome variable: Rate of
weight-gain/kg/day.
Results: There were 49 subjects
in each group. Both groups were comparable. Rate of weight-gain was
found to be (9.59±3.39 g/kg/d) in LRUTF group and (5.41 ± 1.05 g/kg/d)
in locally prepared F100 group. Significant difference in rate of weight
gain was observed in LRUTF group (P<0.0001; 95% CI 3.17-5.19). No
serious adverse effect was observed with use of LRUTF.
Conclusion: LRUTF promotes more
rapid weight-gain when compared with F100 in patients with severe acute
malnutrition during rehabilitation phase.
Key words: Malnutrition, Management, Ready-to-use
therapeutic food, India.
|
|
Guidelines provided by World Health Organization
(WHO) for management of children with severe malnutrition advise two
formula diets, F75 and F100. F75 (75 kcal/100mL) diet is used during
initial phase of treatment while F100 (100kcal/100mL) is used during
rehabilitation phase after appetite has returned [1].These diets can be
prepared locally using cow milk, sugar, vegetable oil, and water.
These diets need to be prepared just before
consumption, as cow milk used can act as growth medium for pathogenic
bacteria if proper hygienic conditions are not maintained. Milk can be
easily adulterated. Shelf-life of locally produced F100 depends on its
constituents like milk which has a very short shelf-life of few hours in
tropical climates [2].
To deal with these problems there was a need to
develop a therapeutic feed which had prolonged shelf-life, was a poor
growth media for pathogens, could be prepared locally with available
resources, was cheap and locally acceptable. A local ready to use
therapeutic food (LRUTF) was prepared from groundnut (25%), milk powder
(30%), sugar (30%), and vegetable oil (15%) by weight. In this study,
efficacy of this LRUTF in promoting weight-gain during rehabilitation
phase was compared with locally-prepared F100 diet.
Methods
All patients aged 6 to 60 months, diagnosed as Severe
acute malnutrition hospitalized in our institution during the study
period (1 October 2009 to 30 May 2010) were included in study. The study
was non-randomized controlled trial. Patients were divided into two
groups depending on the dates of hospitalization. Study was conducted
with permission from hospital authorities.
Severe acute malnutrition was defined as the presence
of severe wasting (<70% weight-for-height or
³3SD) (WHO standards)
[3], bipedal pitting edema of nutritional origin or mid
upper arm circumference (MUAC) of <
11.5 cm in children between 6-60 months of
age [4]. Patient was labelled as uncomplicated if
he was alert, with preserved appetite i.e. appetite test passed,
clinically assessed to be well (absence of general danger signs and
severe anemia, cough and difficult/fast breathing, cold to touch and
severe dehydration), and living in a conducive home environment. All
uncomplicated patients were treated at home and others were
hospitalized.
Appetite test:
Poor appetite was one of the criteria for
hospitalization and inpatient treatment. Appetite was tested with help
of measured quantity of LRUTF (approximately 5g/kg). The idea of doing
appetite test is that, any child who passes appetite test means that he
is able to take ¼ of his maintenance calories at a time, and thus if
four or five equal amounts of feeds are given at home child will not
further lose weight. A child failing in appetite test was hospitalized
[5].
Patients were excluded from study if they refused to
get hospitalized, refused for consent, left against medical advice
before discharge or died during stabilization phase. All children below
age of 6 months with severe acute malnutrition were considered
complicated and hospitalized, but they were excluded from study.
Sample size estimation: Primer of Biostatistics
Ver. 5.0 was used for estimation of sample size based on expected means
in two groups for hypothesis testing. With 5% alpha error, 80% power,
expected difference of means as 2, and expected SD within two groups as
3.4, (calculated from the observations of Diop EHI, et al. [6] )
the minimum sample size was estimated as 47 in each groups. A sample
size of 49 was taken after adjusting for the effect of likely attrition.
Intervention: Upon patient enrolment, informed
written consent was taken from the caregiver. Information about the
history of illness, family demographics, and literacy status of
caregiver was acquired. Appetite test was done using LRUTF. Initial
stabilisation phase was begun after hospitalization, life-threatening
problems were identified and treated, specific deficiencies were
corrected, metabolic abnormalities were reversed and feeding was begun.
During this initial stabilization phase, cautious feeding was begun with
F75. This phase was similar in both cohorts. Once patient showed signs
of improvement (disappearance of fever and other signs of infection,
regaining of appetite, started losing edema) he was shifted into
rehabilitation phase.
All those children who successfully completed
stabilization phase were included in this study. On completing
stabilization phase, children were given a test feeding of the LRTUF and
locally prepared F100 to screen for food allergy and ensure
acceptability. These children were assigned into one of the two groups
by systematic allocation according to order of entry into the study,
with initial participants receiving F 100 (all subjects admitted between
October 1, 2009 to January 31, 2010 ), while children enrolled in later
part of study (between 1 February to 15 May, 2010) received locally
prepared LRUTF .
During rehabilitation phase, children received either
4 meals of F100 or 4 meals of LRUTF daily according to the group
allocation, in addition to 4 meals of food from family pot. Children in
LRUTF group received measured quantity of 12 g/kg/day of LRUTF daily.
Children in F100 group received 60 mL/ kg/day of F100 in 4 quarters.
This therapeutic food provided approximately 60 calories/kg/day.
Patients also received approximately 60 kcal/kg/day by family food.
Thus, a total of 8 feeds per day and around 120 kcal/kg/day with 1-1.5
g/kg of protein were given to every child. All children received
vitamins and mineral supplements as per WHO recommendations [1].
F100 was prepared in lots, quantity of which was
determined by number of children with severe acute malnutrition admitted
at that particular time. It was prepared at 8.00 A.M., 2.00 P.M., 8.00
P.M. and 2 A.M. by one of the investigators. Food from family pot was
consumed at 11.00 A.M., 5.00 P.M., 11.00 P.M. and 5.00A.M. under
observation of an investigator. LRUTF was prepared every Sunday in
hospital kitchen under all aseptic precautions and was stored in sterile
airtight containers of 1kg each. Measured quantity of LRUTF was given
just prior to consumption. Left over LRUTF at the end of day was
discarded and new container was opened each day. Timings of feeding with
LRUTF were similar to those of F100. If child felt hungry in between
meals he was offered family food.
Children were considered ready for discharge when
they were alert and active, eating at least 120-130 kcal/kg/day with
consistent weight gain (of at least 5 g/kg/day for 3 consecutive days)
on exclusive oral feeding, receiving adequate micronutrients, free from
infection, had completed immunization appropriate for age and had gained
at least 15% of admission weight; and the caretaker had been sensitized
to weight gain [4].
Before discharge from hospital, caregiver of each
child was taught to prepare LRUTF and locally prepared F100. They were
advised to give LRUTF and locally prepared F100 at home in same quantity
as in hospital and report every 15 days. Weight gain was calculated
before discharge and on each follow-up. Patients were followed till they
achieved weight <1 SD below mean for height. If a child had poor
weight-gain during follow-up, he was readmitted and treated as secondary
failure. Failure to respond (secondary failure) was indicated by
failure to gain at least 5 gm/kg/day for 3 consecutive days during
rehabilitation phase [1].
Outcome: Primary outcome variable was rate of
weight gain (g)/kg bodyweight/day. This was calculated as follows:
(W2 – W1) ×1000
----------------------
(W1 × N)
Where, W2 – Weight at the time of discharge (kg); W1
– Minimum weight during study period (kg); and N – Number of days from
minimum weight to discharge.
Recipe for F100 and LRUTF: Composition of LRUTF
and F-100 is described in Table I. Production of LRUTF
included grinding, mixing and packaging. Shelled peanuts were roasted in
a roaster at a temperature of approximately 160º C for 40-60 minutes.
This was followed by grinding them into smaller particle sizes in a
grinder such as a hammer mill. Skimmed milk powder, the ground peanuts,
vegetable oil, powdered sugar were then blended in a mixer. The paste
was then homogenized to further reduce particle size (< 200 µm), and
packed [7].
TABLE I Composition of F100 and Locally Prepared F100 Diet and Locally-prepared
Ready-to-use Therrapeutic Food Used in the Study
|
Ingredient |
LRUTF (1kg) |
F 100 (1L) |
|
Fresh cow’s milk |
- |
880 mL |
|
Sugar |
300 g |
75 g |
|
Vegetable oil |
150 g |
20 g |
|
Peanut butter |
250 g |
- |
|
Milk powder |
300 g |
- |
|
Water
|
Nil |
To make 1000 mL |
|
Calories
|
5440 kcal/kg |
1053.8 kcal/L |
|
Proteins
|
136.3 g/kg |
30 g/L |
|
For a child weighing 10 kg
received 120 g/day of LRUTF; i.e. 653 kcal of energy and 16.35 g
of protein. For a child weighing 10 kg received 600 mL/day of
F100; i.e. 632 kcal of energy and 18.4 g of protein.
|
Data analysis: The collected data was entered
into spread sheet programme and analyzed by statistical software Primer
of Biostatistics (Ver. 5.0). The inter-group outcome variables were
analysed by comparing mean and standard deviation in each group.
Unpaired t test was used for hypothesis testing. P<0.05
was considered significant.
Results
During the study period, 118 patients with severe
acute malnutrition were identified, of which 9 patients died during
initial stabilisation phase, 5 patients refused to get hospitalized and
6 patients left before treatment was completed, and were excluded from
the study (Fig. 1). 76 children were in age group of 6
months to 24 months and 22 children were in age group 25 months to 60
months. There were 49 boys (50%). Age and sex distribution in both
cohorts was comparable. 31 (31.6%) patients had edematous malnutrition.
53 (54.1%) patients passed appetite test on admission.
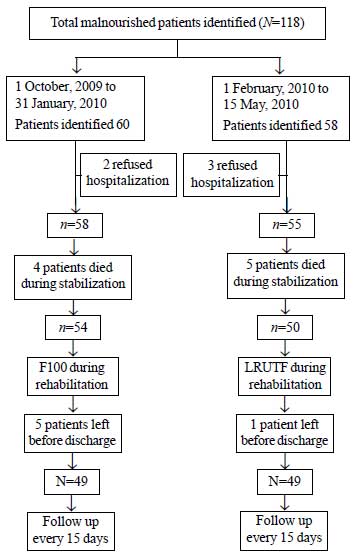 |
|
Fig. 1 Study flow chart.
|
Table II compares the outcome variables
between the two groups. None of the patient in LRUTF group had any
complications related with LRUTF. No patient had peanut allergy.
TABLE II Outcome of Hospitalized Malnourished Children Managed With Locally-prepared
F100 and Locally-prepared Ready-to-Use Therapeutic Food*
|
LRUTF Group (n=49)
|
F100 group (n=49)
|
Mean difference |
|
Weight gain, Mean (SD) |
No. |
Weight gain, Mean (SD)
|
No. |
(95 % CI) |
|
Rate of wt. Gain (g/kg/day) in study
cohort
|
9.59 (±3.39)
|
49 |
5.41 (±1.05) |
49 |
3.174-5.186 |
|
Rate of wt gain (g/kg/day) in
edematous pt |
7.94 (±2.19)
|
15 |
5.10 (±0.88)
|
16 |
1.629-4.051 |
|
Rate of wt gain (g/kg/day) in non
edematous pt |
10.32 (±3.59)
|
34 |
5.66 (±1.10)
|
33 |
3.356-5.964 |
|
Rate of wt gain (g/kg/day) in pt
with good appetite |
10.55 (±3.58) |
28 |
6.06 (±0.85) |
25 |
3.015-5.965 |
|
Rate of wt gain (g/kg/day) in pt with
poor appetite |
8.30 (±2.70) |
21 |
4.73 (±0.78) |
24 |
2.408-4.732 |
|
Rate of wt gain (g/kg/day) on follow up
(g/kg/d) |
9.43 (±2.90) |
16 |
5.22 (±0.84) |
18 |
2.756-5.664 |
|
Duration of hospital stay (days) |
13.04 (±0.16)
|
49 |
16.20 (±4.73) |
49 |
-4.502-1.818 |
|
*P<0.0001 for all measurements;
LRUTF: Locally-prepared Ready-to-Use therapeutic food.
|
Discussion
The results indicate rate of weight-gain is
significantly more with use of LRUTF than F100 during rehabilitation
phase of SAM management. Further, the rate of weight-gain after
discharge from hospital is more with use of LRUTF. Duration of
hospitalization is also significantly less with use of LRUTF. This has
great relevance in treatment of severe malnutrition at the national
level as it can decrease the cost of treatment to a great extent. LRUTF
was well tolerated in all age groups without showing any side-effects.
Major limitation of this study was that children were
not randomly assigned thereby increasing chances of selection bias.
Another limitation was that study was not blinded. There was a practical
difficulty in blinding because of different appearance of the two
therapeutic regimens; one being liquid and other being in powdered form.
Observer bias in study was reduced by the fact that primary outcome
measure of this study was determined by nude bodyweight determined on a
calibrated electronic weighing scale rather than by a more subjective
assessment. No observation was made to confirm whether mothers were
actually feeding their children the recommended amount of LRUTF or F100
rigorously at home. Although no peanut allergy was found in study, this
might not be the case in the general population. Sample size in this
study was small as this study was done as a pilot project.
Ciliberto, et al. [8] conducted a study to
test efficacy of LRUTF and standard WHO treatment (F100) in promoting
weight-gain in children with severe acute malnutrition. Their study was
done in uncomplicated SAM children and on outpatient basis [8]. The rate
of weight-gain in the study was 3.5 g/kg/day in LRUTF group and 2
g/kg/day in other group.
Present study was conducted in complicated SAM
patients who were hospitalized. A similar study in hospitalized patients
by Diop, et al. [6] reported average weight-gains of 15.6 and
10.1 g/kg/d in the RTUF and F100 groups, respectively [6]. In our study,
the average weight gain was 9.59g/kg/day and 5.41 g/kg/day. A systematic
review also suggested that use of therapeutic nutrition products like
RUTF for home-based management of uncomplicated SAM appears to be safe
and efficacious [9].
Although rate of weight-gain in studies mentioned
above was different, but in all these studies, rate of weight gain was
better in LRUTF group versus F100. With good acceptability in the
population, no adverse reactions, and better weight-gain, LRUTF is of
great help in the management of rehabilitation phase of severe acute
malnutrition. Further studies with large sample size and home-based
follow-up should be conducted to assess the feasibility and efficacy of
locally-prepared RUTF in management of SAM.
Acknowledgments: Dr RJ Meshram for
critically reviewing the article and Dr Vijay Bhagat for statistical
analysis of the data.
Contributors: GT and CP prepared and edited the
manuscript as per the journal requirements. HPS planned and supervised
the study and would be the guarantor. The final manuscript was approved
by all authors.
Funding: None; Competing interests: None
stated.
|
What Is Already Known?
• F100 promotes weight gain in rehabilitation
phase of malnutrition treatment.
What This Study Adds?
• Locally-prepared Ready-to-use Therapeutic
Food is better than F100 in promoting weight-gain in
hospitalized children with severe acute malnutrition during
hospitalization and after discharge.
|
References
1. World Health Organization. Management of Severe
Malnutrition: a Manual for Physicians and Other Senior Health workers.
Geneva: WHO; 1999.
2. Brewster DR, Manary MJ, Menzies IS, Henry RL,
O’Loughlin EV.Comparison of milk and maize based diets in kwashiorkor.
Arch Dis Child. 1997;76:242–8.
3. WHO Child Growth Standards. Available from URL:
www.who.int/childgrowth/standards. Accessed on June 28, 2011.
4. WHO Child Growth Standards and the Identification
of Severe Acute Malnutrition in Infants and Children, A Joint Statement
by the World Health Organization and the United Nations Children’s Fund.
Available fromURL:www.who.int/nutrition/publications/en/manage_ severe_
malnutrition_ eng.pdf . Accessed on June 28, 2011.
5. Mother and Child Nutrition, Mother Infant and
Young Child Nutrition & Malnutrition. Available from URL:
http://motherchildnutrition.org/malnutrition-management/info/appetite-test.html.
Accessed on June 28, 2011.
6. Diop EHI, Dossou NI, Ndour MM, Briend A, Wade S.
Comparison of the efficacy of a solid ready-to-use food and a liquid,
milk-based diet for the rehabilitation of severely malnourished
children: a randomized trial. Am J Clin Nutr. 2003;78: 302-7.
7. Manary MJ. Local production and provision of
ready-to-use therapeutic food (RUTF) spread for the treatment of severe
childhood malnutrition. Food Nutr Bull. 2006;27:S83-9.
8. Ciliberto MA, Sandige H, Ndekha MJ, Ashorn P,
Briend A, Ciliberto HM, et al. Comparison of home-based therapy
with ready-to-use therapeutic food with standard therapy in the
treatment of malnourished Malawian children: a controlled, clinical
effectiveness trial. Am J Clin Nutr. 2005;81:864-70.
9. Gera T. Efficacy and safety of therapeutic nutrition products for
home-based therapeutic nutrition for severe acute malnutrition: a
systematic review. Indian Pediatr. 2010;47:709-18.
|
 |
|

