V.V. Khadilkar, A.V. Khadilkar, M. Nandy*, G.B.
Maskati
From the Growth and Pediatric Endocrine Unit,
Hirabai Cowasji Jehangir Medical Research Institute, Jehangir
Hospital, 32, Sassoon Road, Pune 411 00, Maharashtra and *LG Life
Sciences India Pvt. Ltd., LG House, AB-3, Safdarjung Enclave, New
Delhi 110 029.
Correspondence to Dr. Vaman Khadilkar, Consultant
Pediatric Endocrinologist, Hirabai Cowasji Jehangir Medical Research
Institute, Jehangir Hospital, 32, Sassoon Road, Pune 411 001.
E-mail: akhadilkar@vsnl.net,
vkhadilk@vsnl.com
Manuscript received: May 12, 2005, Initial review
completed: June 24, 2005;
Revision accepted: September 23, 2005.
Abstract:
We assessed the effect of one year of
therapy with recombinant Human Growth Hormone (rhGH) on growth
velocity of 16 Indian girls with Turner Syndrome (TS) in a
prospective, open trial. Patients received rhGH in a dose of 1
IU (0.3 mg)/kg/week. The mean pretreatment height was 117.1
cms (Z score –3.4), height velocity was 3.8 cm per year (Z
score –2.4), and predicted height was 140 cm. At the end of
therapy mean height was 123.9 (Z score –3.1), height velocity
was 6.7 cm per year (Z score + 1.7), and the predicted height
was 142.4 cm. The increment in height velocity with growth
hormone therapy was statistically significant (P value =
0.001) and the mean increment in predicted height was 2.4 cm.
Our study shows that girls with TS in India benefit from
therapy with rhGH.
Key words: Growth hormone, Height velocity, Turner
syndrome.
Turner syndrome occurs due to a complete or
partial absence of the second X chromosome in girls and is characterized
by short stature, female phenotype, sexual infantilism and somatic
abnormalities(1). It is seen approximately in 1 in 2000 female live
births. Short stature is seen in more than 95% of patients with Turner
syndrome (TS) and they are likely to have a mean adult height of up to
20 cm less than that of the general female population(2). As such a
significant focus of medical management in TS is on growth promoting
strategies. In recent years a number of studies have indicated that the
administration of recombinant Human Growth Hormone (rhGH) can increase
the growth velocity of girls with TS(3). We report here the results of a
study where 16 girls with TS were treated with rhGH for a period of one
year.
Subjects and Methods
A prospective, open label trial with rhGH was
performed at five centers in 16 girls with TS who had never received
rhGH. The diagnosis of TS was confirmed by Karyotype. Chronological age
of the girls ranged from 7.2 to 17.1 years (mean 11.1 years) and
skeletal age ranged from 4.9-13.5 years (mean 9.4 years). The ethics
committee of all the hospitals approved the study and informed consent
was obtained from all parents. The inclusion criteria–girls with
confirmed diagnosis of TS, with a height of <–2 standard deviation (SD)
below the population mean, having at least one previous height reading
within the past 3 months, euthyroid status (or controlled on
medication). Patients with known Growth Hormone resistance, or any
abnormality likely to affect growth or its evaluation such as renal
insufficiency were excluded. Height was recorded using a Child Growth
Foundation Stadiometer to the accuracy of 1 mm, while weight was
recorded on a Salter electronic scale to an accuracy of 100 grams.
At enrollment height, weight, parent’s height,
previous height measurement, pre-existing conditions and concomitant
medications were recorded. A detailed physical examination was
performed, an X-ray of the non dominant wrist and hand for bone
age was taken and blood was drawn for a hemogram, fasting blood glucose,
glycosylated hemoglobin, calcium, phosphorous, alkaline phosphatase,
serum electrolytes, blood urea, serum creatinine, AST, ALT, free T4 and
free T3, thyroid stimulating hormone (TSH) and insulin like growth
factor 1 (IGF-1).
All patients received growth hormone in a dose of 1
IU (0.3 mg/kg/week) given as seven divided doses as subcutaneous daily
injection at night. Growth hormone was provided by LG lifesciences (Eutropin)
as 4 IU vials. The 4 IU vial contains 1.33 mg of lyophilized recombinant
rhGH protein and a separate vial contains 1 mL solvent for solution. The
protein consists of 191 amino acid residues and is produced from
genetically engineered yeast cells of the strain Saccharomyces
cerevisiae. All patients were asked to maintain a diary and missed
doses, local and systemic reactions were recorded. Height and weight
measurements were performed again at 6 months and at the end of one year
of therapy. Blood investigations were repeated after one year of
therapy. All sixteen patients completed the one-year study period.
Data analysis was performed using Microsoft excel
2000 data analysis pack. Height, weight, height velocity, body mass
index (BMI), mid parental height (MPH) and IGF-1 were expressed as
standard deviation scores (Z scores)(4,5). TW3 RUS method was used for
calculating the bone age and predicted height of children based on their
current age, height and RUS score(7). Student’s t-test was used
to compare the difference between means.
Results
Of the 16 patients studied, five had a Karyotype of
45 XO, eight were 45X/46XX and three were 45XX (iXq) and none of the
girls showed signs of puberty. The mean pretreatment parameters are
depicted in Table I. At the beginning of the treatment, all
patients thyroid function, creatinine, hemoglobin, electrolytes, blood
sugar, glycosylated hemoglobin, calcium, and liver function tests were
normal and were free from any major systemic disease except the
abnormalities associated with Turner syndrome. The associated conditions
were scoliosis and kyphoscoliosis, squint, horseshoe kidney, coarctation
of aorta, lymphedema, anemia, difficulty in mathematics at school and
one patient developed hypothyroidism while on treatment with rhGH. One
patient reported urticaria during the first month of therapy, which
subsided without anti-allergy treatment. No other local or systemic
reactions possibly occurring due to growth hormone administration were
noted.
Table I
Growth Parameters in Children with Turner’s Syndrome Before and After Therapy.
|
Parameter |
Pretreatment |
Post-treatment |
|
Age (years) |
11.1 (2.8) |
12.1 (2.8) |
|
Height (cm) |
117.1 (11.5) |
123.9 (11.3) |
|
Height velocity (cm/year) |
3.8 (1.3) |
6.8 (1.4) |
|
Weight (kg) |
22.6 (7.7) |
25.8 (8.6) |
|
BMI (kg/m2) |
16.1 (2.6) |
16.4 (2.9) |
|
Bone age (years) |
9.6 (2.9) |
10.6 (2.8) |
|
Height Z score |
–3.4 (1.1) |
–3.1 (1.2) |
|
Height velocity Z score |
–2.4 (2.5) |
+1.7 (2.4) |
|
Weight Z score |
–1.5 (0.7) |
–1.4 (0.9) |
|
BMI Z score |
–0.4 (2.9) |
–0.5 (0.9) |
|
Predicted height |
140.0 (6.4) |
142.4 (7.0) |
All values are expressed as mean (SD).
Post-treatment parameters at the end of one year of
therapy are listed in Table I.
The mean increment in bone age was 1year at the end
therapy. The increment in height velocity with growth hormone therapy
was statistically significant (P = 0.001) (Fig.1). Patient’s
height had a strong correlation with the mid-parental height
(correlation coefficient = 0.7). The mean increment in predicted height
at the end of therapy was 2.4 cm. The mean alkaline phosphatase was
381.1 U/L at start of therapy and rose to 543 after one year of therapy.
The IGF-I Z scores rose from -0.6 to + 2.5 and the difference was
statistically significant (P = 0.03).
|
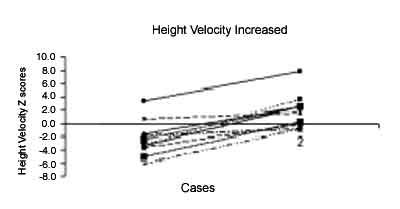 |
|
Fig. 1. Improvement in height velocity in 15
patients with Turner syndrome while on a year of therapy with
Growth hormone. |
Using Ranke’s data for girls with Turner Syndrome the
pretreatment height Z score of our Turner girls was –1.1, and after
treatment it was –0.51(8). The pretreatment height velocity Z score as
compared with Ranke’s data was +0.55 while after treatment with growth
hormone this was + 4.31.
Discussion
The diagnosis of TS should be suspected in any girl
who presents with unexplained short stature, even in the first 3 years
of life(9). The exact etiology of short stature in TS is still a subject
of speculation and the hypotheses include a gene located in the
pseudoautosomal region (PAR 1) at the tip of the short arm of the X
chromosome (Xp 22.3), global genetic factors, and lack of pubertal
growth(10,11). A strong correlation between TS patients’ height and
mid-parental height (MPH) has been confirmed in several studies(11,12).
Clinical trials of recombinant rhGH therapy have
shown that rhGH accelerates the linear growth rate. In a landmark trial
Rosenfeld, et al.(13) who followed their patients until the age
of 17-18 (near final height) showed that patients treated with rhGH
gained 8.5 cm over their projected final height. These and other similar
studies have shown that a final height of 150 cm is an achievable goal
in TS girls with the use of rhGH. Growth Hormone treatment if started
early, results in normalization of height during childhood and
normalization of adult height in most of the girls treated with rhGH for
a number of years(3). The recommended starting dose of rhGH is around
0.15 IU (0.05 mg/kg) per day which is higher than the dose used for
children with Growth Hormone Deficiency. Growth hormone therapy is
usually well tolerated. The potential untoward reactions are occurrence
of benign intracranial hypertension, carbohydrate intolerance, edema,
local reactions such as redness and itching, slipped capital femoral
epiphyses and exaggeration of scoliosis(1).
In India the diagnosis of TS is often delayed not
only because of a lack of expertise but also because the girl child is
neglected and brought to the attention of medical help because of
pubertal delay rather than short stature. Second major problem is the
enormous cost of rhGH therapy, which is in the range of 3-5 lakh
rupees/year.
In our study there was a significant increase in the
height velocity during therapy showing that rhGH can stimulate
short-term growth in patients with TS. The improvement in the predicted
height after one year of rhGH therapy was 2.4 cm, similar to the
improvement in predicted height shown in other studies(13). Western data
suggests that TS girls have a higher BMI and are overweight as compared
to the general population. We did not find this in our patients; this
could be due to poor nutrition or a different phenotype. There was a
mean increment of one year in the bone age during rhGh therapy
confirming that growth hormone selectively promotes height growth
without advancing bone age thus improving the final height. When a
comparison was made between Indian TS girls and Ranke’s TS standards our
patients were generally shorter but were growing at normal speed for TS
before treatment with rhGH was started.
Our study shows that Indian girls with Turner
syndrome benefit from rhGH therapy, demonstrating improved short-term
skeletal growth as well as improved final height prediction.
Acknowledgements
We thank Dr. Madhulika Kabra, Additional Professor,
All India Institute of Medical Sciences, New Delhi, Dr. Mala
Dharmalingam, Associate Professor, M.S. Ramaiah Medical College and
Hospital, Bangalore, Ms. Deepa Lokhandi and the nursing and pharmacy
staff of the respective hospitals for their valuable assistance in
successful completion of the study.
We would also like to extend our sincere gratitude to
LG Life Sciences India for their generous supply of human growth
hormone.
Contributors: VVK and AVK carried out the
clinical workup. AVK, MN and GBM collected and verified the data and
drafted the manuscript. VVK will act as guarantor of the study.
Funding: LG Life Sciences India Pvt. Ltd., LG
House, AB-3, Safdarjung Enclave, New Delhi 110 029 and HCJMRI, Jehangir
Hospital, Pune.
Competing Interests: Financial support for the
investigations and the growth hormone was provided by LG Life Sciences
India Pvt. Ltd., at all centers. M Nandy, One of the authors, is also an
employee of LG Life Sciences India Pvt. Ltd.
|
Key Messages |
|
• Short stature is seen in more than 95% of patients with Turner
syndrome and hence a significant focus of medical management in
Turner Syndrome is on growth promoting strategies.
• Indian girls with Turner syndrome benefit
from recombinant human growth hormone therapy demonstrating
improved growth velocity and final height prediction.
|
|
|
1. Shah N. Turner Syndrome. In: Desai
MP, Bhatia V, Menon PS, editors. Pediatric Endocrine Disorders.
1st ed. Hyderabad: Orient Longman; 2001, p. 169-180.
2. Theo CJ, Sabine MP, Theo S, Maarten J,
Barto JO, Thomas V, et al. Normalization of height in
girls with Turner syndrome after long-term growth hormone
treatment: Results of a randomized dose-response trial. J Clin
Endocrinol Metab. 1999; 84: 4607-4608.
3. Rosenfeld RG, Brasel JA, Burstein S,
Chernausek SD, Johanson AJ. Growth hormone therapy of Turner’s
Syndrome: beneficial effect on adult height. J Pediatr 1998;
133: 803-804.
4. Agarwal DK, Agarwal KN, Upadhyay SK,
Mittal R, Prakash R, Rai S. Physical and sexual growth pattern
of affluent Indian children from 5-18 years of age. Indian
Pediatr 1992, 29: 1203-1282.
5. Tanner JM, Whitehouse RH, Takaishi M.
Standards from birth to maturity for height, weight, height
velocity and weight velocity; British children 1965. Arch Dis
Child 1966; 41: 454-471.
6. Dehiya RD, Bhartiya D, Kapadia C, Desai
MP. Insulin Like Growth Factor-1, Insulin Like Growth Factor
Binding Protein-3 and acid labile subunit levels in healthy
children and adolescents in mumbai suburbs. Indian Pediatr 2000;
37: 990-997.
7. Tanner JM, Healy MJ, Goldstein H, Cameron
N. In: Assessment of Skeletal Maturity and Prediction of
Adult Height (TW3 method). 3rd ed. London: WB Saunders, 2001.
8. Ranke MB. Spontaneous growth in turner’s
syndrome. Acta Pediatr Scand 1988; 343: 22-30.
9. Davenport ML, Punyasavatsut N, Stewart PW,
Gunther DF, Savendahl L, Sybert VP. Growth failure in early
life: an important manifestation of Turner syndrome. Horn Res
2002: 57: 157-164.
10. Rao E, Weiss B, Fukami M, Rump A, Niesler
B, Mertz A. et al. Pseudoautosomal deletions encompassing
a novel homeobox gene cause growth failure in idiopathic short
stature and Turner syndrome. Nat Genet 1997; 16: 3-4.
11. Kosho T, Muroya K, Nagai T, Fujimoto M,
Yokoya S, Sakamoto H, et al. Skeletal features and growth
patterns in 14 patients with haploinsufficiency of SHOX:
Implications for the development of Turner syndrome. Endocrinol
Metab 1999; 84: 4613-4621.
12. Cohen A. Kauli R. Pertzelan A. Final
height of girls with Turner’s syndrome: correlation with
karyotype and parental height. Acta Pediatr Scand 1995: 84:
550-554.
13. Rosenfeld RG. Hintz RL, Johanson AJ, Breasel JA, Burstein
S. Chernausek SD, et al. Methionyl human growth hormone
and oxandrolone in turner syndrome: preliminary result of a
prospective randomized trial. J Pediatr 1986; 109: 936-943.
|