Diarrhea is a leading cause of death in
children accounting for 9% of all deaths among children under-5
year worldwide in 2015
[1] and an estimated 300,000 children in India each year
[2]. Dehydration is associated with deaths in most cases [3] and
occurs when fluid losses are not replaced adequately and a
deficit of water and electrolytes develops. The total body
sodium deficit in diarrheal dehydration in young children is
about 70-110 millimoles per liter of water deficit. Potassium
and chloride losses are in a similar range [3]. The preferred
regime for treatment of children with severe dehydration is
rapid intravenous rehydration using World Health Organization
(WHO) Plan C. WHO recommends use of Ringer lactate or normal
saline in case of non-availability of Ringer lactate, for
intravenous rehydration in children under plan C [3].
METHODS
This equivalence randomized control trial was done in the
Department of Pediatrics, Maulana Azad Medical College and
associated Lok Nayak Hospital, New Delhi during the period May,
2016 – April, 2017. The study was approved by Institute ethics
committee. Children between 1 to 12 years of age with acute
diarrhea and severe dehydration were enrolled after taking
informed consent from their parents. Acute diarrhea was defined
as
³3 loose stools in previous 24 hour and duration of
diarrhea less than 14 days. Severe dehydration was defined as
per WHO guidelines [3] with two or more of the following:
lethargic or unconscious, drinks poorly or not able to drink,
skin pinch goes back very slowly (>2 second) and sunken eyes.
Children with dysentery, severe acute malnutrition (WHO
criteria), severe anemia (significant palmar pallor),
meningitis, seizures, known surgical problems (e.g.
ileostomy), known systemic disease and hypoglycemia (Blood
glucose <54 mg/dL) were excluded. Eligible children were
randomly assigned to receive either Ringer lactate or normal
saline (Fig. 1). Allocation sequence was computer
generated (www.randomization.com) and allocation
concealment was done through serially numbered opaque sealed
envelopes (SNOSE).
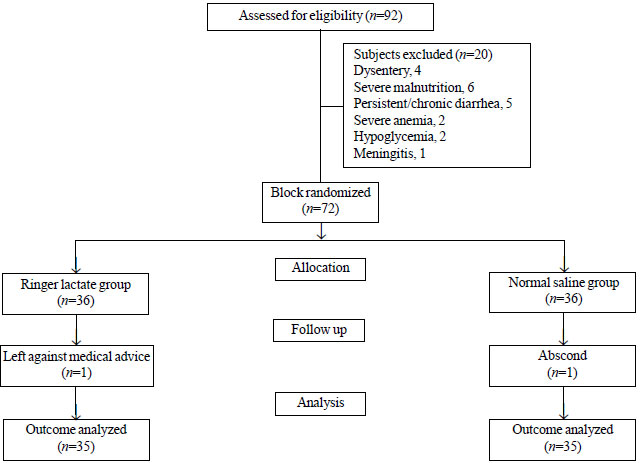 |
| Fig. 1 Flow diagram
of patients. |
Before commencement of rehydration, blood samples
were taken for blood gas analysis, kidney function tests and
serum electrolytes (sodium and potassium). Hyponatremia was
defined at serum sodium <135 mmol/L. Children received Ringer
lactate or normal saline according to WHO guidelines in doses of
100 mL/kg over 3 hour and were monitored every 30-60 minutes for
vital signs. They were reassessed at the end of 100 mL/kg
infusion for clinical signs of dehydration. Caregivers were
asked to mark the number of stool purges and the number of
vomiting for the correction period. If any child was found in
dehydration at the end of first correction, the child was
treated according to standard WHO guidelines. At the end of
first correction, blood samples were repeated for blood gas,
renal function and serum electrolytes. In initial hours ongoing
losses were replaced by intravenous fluid solution of 0.45%
saline in 5% dextrose and 20 mEq/L potassium chloride at 10
mL/kg per loose stool at hourly intervals. Children also
received age appropriate maintenance fluids. All children
received oral elemental zinc supplementation at 20 mg/day.
Completion of first fluid correction at 3 hour was taken as
primary end point and disappearance of all clinical signs of
dehydration was taken as endpoint for secondary outcome. Our
primary objective was to determine the difference in the change
of serum sodium level over baseline in the two groups. We also
studied the difference in the change of serum potassium, pH,
bicarbonate levels and base deficit at primary end point. The
time taken and volume of fluid requirement for complete
rehydration in the two groups were compared at secondary end
point.
Sample size was calculated to demonstrate
equivalence between the two interventions with an equivalence
limit not exceeding 3 mEq in serum sodium level with SD of 3, a = 1% and power of 90. A sample size of 30 children
was calculated. Expecting 20% attrition, 36 subjects were
enrolled in each group in an age stratified manner in 2:1 ratio
for age groups 1-5 years and >5-12 years.
Statistical analyses:
Analysis was conducted using IBM SPSS Statistics (version 22.0).
The normality of quantitative data was checked by measures of
Kolmogorov-Smirnov tests of normality. For primary outcome, the
two groups were compared for change in serum sodium from
baseline. Means of two groups were compared using independent
t-test. Mann-Whitney U-test was carried out for statistical
analysis of skewed continuous variables. For comparison of
normally time related variables paired t-test was applied.
Proportions were compared using Fisher’s exact test. All the
statistical tests were two-sided and were performed at a
significance level of 0.05.
Results
Out of 72 enrolled children, 70 (35 in each group) completed the
therapy. One child in each group opted out of the study before
the first correction of dehydration. The baseline
characteristics of patients are shown in Table I.
Hyponatremia was present at baseline in 26 (74%) in Ringer
lactate group and 25 (71%) in normal saline group. No child had
symptomatic hyponatremia. Mean (SD) serum sodium values at baseline were comparable (131.3 (4.4) mEq/L in Ringer
lactate group and 132.3 (4.8) mEq/L in normal saline group, P=0.29).
The change in biochemical parameters at primary end point are
depicted in Table II.
Table I Baseline Characteristics of Children With Severe Dehydration Receiving Ringer Lactate or
Normal Saline for Rehydration
|
Characteristics |
Ringer Lactate |
Normal Saline |
|
(n=35) |
(n=35) |
|
Age (y) |
4.3 (2.9) |
4.7 (2.9) |
|
Male |
16 (46) |
17 (49) |
|
Duration of symtoms, d |
1.8 (1.6) |
1.6 (1.4) |
|
*Sodium, mEq/L |
131.3 (4.4) |
132.3 (4.0) |
|
*Potassium, mEq/L |
3.8 (0.6) |
3.5 (0.7) |
|
Blood urea, mg/dL |
53 (35.8) |
59.6 (28.6) |
|
Creatinine, mg/dL |
1.2 (0.7) |
1.3 (0.7) |
|
pH |
7.26 (0.07) |
7.28 (0.08) |
|
Bicarbonate, mEq/L |
12.66 (3.33) |
12.16 (2.89) |
|
Base deficit, mmol/L |
12.58 (3.98) |
12.89 (3.66) |
|
*Serum values; data represented as Mean (SD); P >0.05 for all comparisons. |
Table II Change in Biochemical Parameters During Correction of Severe Rehydration
|
Parameters |
Ringer Lactate (n=35) |
Normal Saline (n=35) |
P value* |
|
Baseline |
After |
Mean (SD) |
Baseline |
After |
Mean (SD) | |
| |
correction |
difference | |
correction |
difference | |
|
Sodium (mEq/L) |
131.3 (4.4) |
132.7 (3.5) |
1.4 (4.5) |
132.3 (4.0) |
134.5 (4.5) |
2.1 (4.9) |
0.58 |
|
Potassium (mEq/L) |
3.8 (0.6) |
3.6 (0.6) |
0.2 (0.4) |
3.5 (0.7) |
3.3 (0.7) |
0.2 (0.5) |
0.60 |
|
Blood urea (mg/dL) |
53 (35.8) |
42.7 (28.6) |
10.3 (18.2) |
59.6 (28.6) |
40.0 (15.8) |
19.6 (21.9) |
0.6 |
|
Creatinine (mg/dL) |
1.2 (0.7) |
0.9 (0.5) |
0.3 (0.3) |
1.3 (0.7) |
0.8 (0.3) |
0.4 (0.5) |
0.42 |
|
pH |
7.26 (0.07) |
7.33 (0.08) |
0.07 (0.05) |
7.28 (0.08) |
7.30 (0.09) |
0.02 (0.07) |
0.002 |
|
Bicarbonate (mEq/L) |
12.66 (3.33) |
15.92 (4.04) |
3.25 (2.14) |
12.16 (2.89) |
13.19 (2.41) |
1.03 (2.66) |
<0.001 |
|
Base deficit (mmol/L) |
12.58 (3.98) |
8.85 (4.48) |
3.73 (2.48) |
12.89 (3.66) |
11.67 (3.66) |
1.22 (2.80) |
<0.001 |
|
All values in mean (SD); *P value for delta difference between both groups. |
After first volume correction (WHO plan C), 23
(65%) children in Ringer lactate group and 17 (49%) children in
normal saline group had persistent hyponatremia, one child had
symptomatic hypokalemia in the latter group, which responded to
standard therapy. A total of 29 (83%) children were completely
rehydrated in each group while 6 (17%) had features of some
dehydration and required Plan B. No child required subsequent
rehydration. Time to rehydration was similar (range 3h-7h) in
both groups. The mean (SD) fluid requirement for replacement of
ongoing losses was similar in both the groups, 74.29 (35) mL/kg
and 76.29 (34.8) mL/kg in Ringer lactate and Normal saline
groups, respectively (P=0.81).
Discussion
In this study, high rate of hyponatremia was detected in
children with acute diarrhea and severe dehydration which
persisted after rehydration. The change of serum sodium was
similar with use of either Ringer lactate or normal saline for
rehydration.
The open label nature of the trial and the
non-availability of serum chloride levels and non-utilization of
oral rehydration solution for replacement of ongoing losses were
the limitations of the study. The study was not powered to
detect significant changes in pH, bicarbonate
and base excess.
In a similar study by Mahajan, et al. [9],
the change in serum sodium levels was similar after rapid
intravenous rehydration with Ringer lactate or normal saline in
children with acute diarrhea. The decline in serum potassium
from baseline in both groups was comparable in the present study
unlike seen only in normal saline group in the earlier study
[9], which was attributed to the composition of normal saline,
which does not have potassium as a constituent. The present
study had lesser metabolic acidosis in comparison to the
previous study [9], which could explain the greater fall in the
potassium levels in their study. In the present study, the
significant changes in pH, bicarbonate and base deficit in Ringer lactate group as compared to normal
saline group can be explained by the conversion of lactate to
bicarbonate in the former group. Similar results were observed
in an adult study [10], unlike Mahajan, et al. [9] where both groups showed comparable change,
which was attributed to intravascular volume expansion.
To conclude, normal saline is equivalent to Ringer
lactate solution in terms of change of serum sodium and serum
potassium from baseline for initial rapid intravenous
rehydration in children with acute diarrhea and severe
dehydration. Rehydration with normal saline does not cause
hypernatremia. Although, quicker resolution of metabolic
acidosis occurs with Ringer lactate solution, its clinical
significance may need to be studied further.
Ethical Clearance:
Institutional Ethics Committee for Human Research, Maulana Azad
Medical College; No. 11/IEC/MAMC/2015/317.
Contributors credit:
MN,APD,RS: involved in execution of the study, data analysis and
writing the manuscript; TKM: contributed in execution of the
study, data analysis and writing the manuscript.
Funding:
None; Competing interest: None stated.
|
What This Study Adds? |
Ringer lactate and normal saline
are equivalent in terms of change in serum sodium from
baseline for rapid intravenous rehydration in children
with acute diarrhea.
|
References
1. Diarrhoea remains a leading
killer of young children, despite the availability of a simple
treatment solution.
UniceF 2017. Available from:
https://data.unicef.org/topic/child-health/diarrhoeal-disease/#.
Accessed April 15, 2017.
2. Lakshminarayanan S,
Jayalakshmy R. Diarrheal diseases among children in India:
Current scenario and future perspectives. J Nat Sc Biol Med.
2015;6:24-8.
3. Bhan MK, Mahalanabis D,
Pierce NF, Rollins N, Sack D, Santoshum M. The treatment of
diarrhoea: A manual for physicians and other senior health
workers. 4th rev. Geneva: World Health Organization; 2005.
4. World Health Organization.
Cholera annual report 2006: Weekly epidemiological record. World
Health Organ 2007;82:273-84.
5. Reid F, Lobo DN, Williams
RN, Rowlands BJ, Allison SP. Normal saline and physiological
Hartmann’s solution: a randomized double-blind crossover study.
Clin Sci (Lond). 2003;104:17-24.
6. Cho YS, Lim H, Kim SH.
Comparison of lactated Ringer’s solution and 0.9% saline in the
treatment of rhabdomyolysis induced by doxylamine intoxication.
Emerg Med J. 2007;24:276-80.
7. Hadimioglu N, Saadawy I,
Saglam T, Ertug Z, Dinckan A. The effect of different
crystalloid solutions on acid-base balance and early kidney
function after kidney transplantation. Anesth Analg.
2008;107:264-9.
8.
O’Malley CM, Frumento RJ, Hardy MA, Benvenisty AI,
Brintjens TE, Mercer JS, et al. A randomized, double
blind comparison of lactated ringer’s solution and 0.9% saline
during renal transplantation. Anesth Analg. 2005; 100:1518-24.
9.
Mahajan V, Sajan SS, Sharma A, Kaur J. Ringer’s lactate
vs normal saline for children with acute diarrhea and severe
dehydration: A double blind randomized controlled trial. Indian
Pediatr. 2012;49:963-8.
10. Cieza JA, Hinostroza J, Huapaya JA, León CP. Sodium chloride
0.9% versus lactated Ringer in the management of severely
dehydrated patients with choleriform diarrhea. J Infect Dev
Ctries. 2013;7:528-32.

