Metronomic chemotherapy is the frequent administration of
chemotherapeutic drugs at doses significantly below the
‘maximum tolerated dose’ with no prolonged drug-free breaks;
it has carved a niche in modern pediatric oncology practice,
especially in the recurrent metastatic or progressive
disease settings [1,2]. Powerful and reliable biomarkers are
yet to be identified and validated for the selection of a
metronomic regimen for a given patient, in a given clinical
setting.
Vascular endothelial growth factor (VEGF) is an in vivo
proangiogenic cytokine while Thrombospondin-1 (TSP-1) is an
intrinsic anti-angiogenic cytokine. Studies have shown
increase in VEGF levels during successful therapy with
anti-VEGF monoclonal antibodies and tyrosine kinase
inhibitors (TKI)
[3,4].
Even though evidence for these
cytokines is contradictory [5-8], these angiogenic peptides
are attractive bio-markers because of their ease of sampling
and estimation in clinics. We previously published a
randomized trial in pediatric metronomics comparing
metronomic chemotherapy with placebo in progressive
pediatric malignancies [9]. In this report, we present the
planned secondary objective of the study wherein we did a
comparative analysis of two angiogenic peptides between
these two groups of patients at different time-points.
METHODS
The design,
setting, participants and methodology of the clinical study
have been described elsewhere [9]. Eligible patients (n=108)
underwent 1:1 simple centralised randomization to metronomic
chemotherapy (4-drug regimen of daily celecoxib and
thalidomide with alternating periods of etoposide and
cyclophosphamide) and placebo groups.
After informed consent, blood
samples were taken for biomarker evaluation at baseline (A1)
and interim assessments (A2 = 9 weeks or earlier if
progressed, A3 = 18 weeks or earlier if progressed) (Fig.
1).
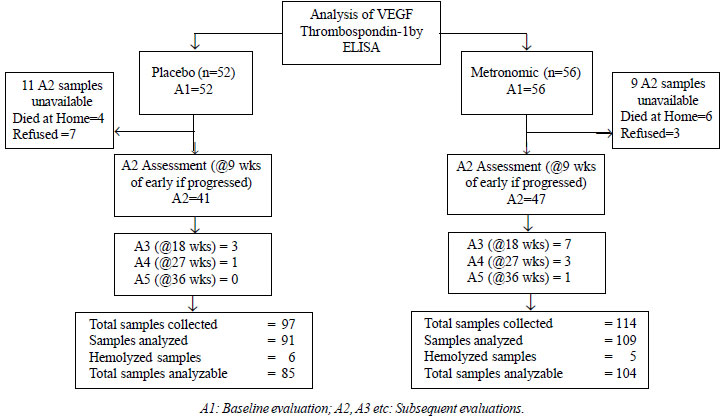 |
| Fig. 1
Study flow diagram. |
Serum was separated and centrifuged
at 1000 g for 10 min within 30 min from collection. Serum
was aliquoted and stored at –80°C. ELISA for VEGF and TSP-1
levels were evaluated from these samples of serum using
Quantikine Human VEGF Immunoassay DVE00 and Quantikine Human
Thrombospondin-1 Immunoassay DTSP10, respectively (R&D
Systems, Inc, Minneapolis, MN 5541 USA).
We analyzed pattern of VEGF and TSP-1 in both study arms,
comparing them at baseline, at second assessment (A2) and at
third assessment (A3) as well as the change in their levels
at A2. The clinical assessment during the study had shown no
significant difference in Progression free survival (PFS) or
Overall survival (OS) between the two arms [9]. However, in
post hoc subgroup analysis, those who had completed more
than 3 cycles (i.e. 9 weeks) and those who did not
have a bone sarcoma benefitted from metronomic chemotherapy
[9]. Hence, we also analyzed the patients as responders
versus non-responders, defining responders as those who
had completed 9 weeks of therapy.
RESULTS
The baseline characteristics of the 108 recruited subjects are
presented in Table I. Baseline levels of VEGF
greater than mean value of 1135.45 pg/mL was found to
adversely affect OS with hazard ratio of 1.77 (1.18-2.65) (P=0.006).
Baseline TSP-1 did not affect OS [HR (95% CI) =0.99
(0.99-1.00) (P=0.92)].
Table I Comparison of Baseline Characteristics of the Two Study Groups
| Characterstics | Placebo | Metronomic |
| (n=52) | (n=56) |
| Age, y* | 15 (5-18) | 13 (5-18) |
| Male: female | 3.3:1 | 3:1 |
| ECOG-PS | | |
| 0 | 1 (1.9) | 3 (5.3) |
| 1 | 19 (36.5) | 18 (32.1) |
| 2 | 21 (40.3) | 25 (44.6) |
| 3 | 11 (21.1) | 10 (17.8) |
| Diagnosis | | |
| Bone Sarcoma (PNET/ | 32 (61.4) | 40 (71.3) |
| Osteosarcoma) | | |
| Neuroblastoma | 5 (9.6) | 5 (8.9) |
| RMS | 6 (11.5) | 3 (5.3) |
| Esthesioneuroblastoma | 1 (1.9) | 1 (1.7) |
| STS | 4 (7.6) | 2 (3.8) |
| Others | 3 (5.7) | 3 (5.3) |
| Retinoblastoma | 1 (1.9) | 2 (3.8) |
| Previous lines | | |
| 2 | 48 (92.3) | 53 (94.6) |
| 3 | 4 (7.7) | 2 (3.6) |
| 4 | 0 | 1 (1.8) |
| All P values >0.05; PNET: primitive neuroectodermal tumours; RMS: rhabdomyosarcoma; STS: soft tissue sarcoma; ANC= absolute neutrophil count; ECOG-PS: Eastern Cooperative Oncology Group- Performance Status. All values in no. (%) except *median (range). |
Mean level of VEGF and TSP-1 in patients at baseline, at A2
and at A3 were
not different in the placebo and metronomic groups (Web
Table I). The difference from baseline
values to second assessment (A2) for both these biomarkers
in each group was also not significantly different.
In the metronomic arm, responders (i.e. those who
completed at least 9 weeks of chemotherapy) had a
significantly lower baseline VEGF levels as compared to
non-responders (P=0.002). However, there was no
difference in TSP-1 levels between them. The mean difference
from baseline to the second assessment (A2-A1) for TSP-1 was
significantly different (P=0.04); while TSP-1
decreased in the responders, it increased in the
non-responders. Such a difference was not noted for VEGF (Table
II). There was no significant difference in the baseline
levels of VEGF and TSP-1 between responders and
non-responders of placebo arm. Neither was there any
significant difference in the mean change of both VEGF and
TSP-1 from baseline to A2 (Table II).
Table II Comparison of VEGF and TSP-1 Levels Among Responders and Non-responders of Metronomic and
Placebo Arms of the Study
| | Metronomic (n=56) | Placebo (n=52) |
| Responders | Non-responders | Responders | Non-responders |
| (n=21) | (n=35) | (n=19) | (n=33) |
| Baseline TSP-1 (A1) | 19.4 (7.0) | 21.3 (10.6) | 24.3 (13.4) | 20.9 (12.5) |
| Baseline VEGF (A1) | 659.7 (362.1)* | 1143.9 (622.0)* | 961.9 (496.3) | 1238.5 (770.1) |
| Difference from baseline to A2 (TSP-1): (A2-A1) | –4.43 (8.0)# | 1.7 (11.3)# | –4.90 (16.4) | –6.2 (12.2) |
| Difference from baseline to A2 (VEGF): (A2-A1) | 173.1 (618.2) | 90.8 (706.1) | 224.9 (615.7) | –87.0 (535.9) |
| All values in mean (SD); VEGF: Vascular Endothelial Growth Factor, TSP-1: Thrombospondin-1, A2= second assessment at 9 wks or earlier if progression of disease; values of VEGF are in pg/mL and the values of TSP-1 are in µg/mL respectively, P value of *0.02 and #0.04. |
Discussion
Our study showed that baseline VEGF predicted OS for the
entire study population, whereas baseline TSP-1 did not
predict the same. In the total study sample, there was no
difference in the levels of VEGF or TSP1, neither at
baseline, nor at any other time-point, between the placebo
and metronomic arms. The magnitude of change from baseline
to A2 was also not different significantly different between
the two arms. But then, there was no difference in survival
as well between the two arms.
While our findings are in contrast to studies on other solid
tumors treated with anti-angiogenic agents, eg.
metastatic colorectal cancer treated with bevacizumab [10],
it corroborates with the findings in metastatic breast
cancer [11]. When we focussed our analysis on the metronomic
arm, we found that responders had significantly lower
baseline levels of VEGF but no difference was noted in
TSP-1. This is consistent with previous studies that have
noted an aggressive tumor progression with injection of
TSP-1 in preclinical models [12].
Although, our study demonstrated some trends, we
could not provide proof of the principle that the 4-drug
anti- angiogenic chemotherapy actually acts by altering the
cytokine milieu of pro and anti angiogenic factors, and
inhibiting angiogenesis in vivo. Our results are
consistent with the results Stempak, et al. [5] and
Kesari, et al. [8] who found that none of the four
tested markers (VEGF, bFGF, endostatin, and TSP-1) were of
prognostic significance.
In a previous study, baseline TSP-1
levels appeared to correlate with prolonged response; this
conclusion was based on just three patients who had a
baseline high TSP-1 level and did not progress for more than
a year [6]. In another study of 100 patients treated with
metronomic chemotherapy, 52 baseline patient samples were
available and herein serum TSP-1 levels increased in
patients who completed therapy than in non- completers [7].
Our study is a larger study with a placebo arm, but still we
could not replicate those findings. The reason why we could
not demonstrate a trend in these cytokines may probably be
the fact that the small subset of proteins that we selected
is unlikely to be representative of the overall effect of
all of the regulators of angiogenesis. Angiogenesis is a
complex interacting cascade of pathways with an interplay of
a large number of proteins inside and outside of the cell
and we cannot gauge them by relying on only one or two of
these proteins. The strengths of our study are its
randomized nature and comparison with placebo.
Identifying reliable predictive
and/or prognostic biomarkers for anti-angiogenic therapies
has been unsuccessful to date. Looking for a biomarker for a
therapy can be a realistic objective only if that therapy
targets the tumor cells of interest, but when we are using
metronomic chemotherapy, we are actually targeting the host
endothelial cells and not directly the tumor. So, it is
unlikely that universal mechanistically-driven markers will
ever be unveiled for metronomic chemotherapy, especially
given its varied mechanisms of action, multiple drug
combinations and many clinical settings. We suggest that
other biomarkers be explored for measuring the efficacy of
metronomic chemotherapy like circulating cell free DNA,
circulating endothelial cells, and circulating endothelial
precursor cells and micro-particles.
Funding:
None; Competing interests: None stated.
WHAT THIS STUDY
ADDS?
Vascular endothelial growth
factor (VGEF) and thrombaspondin-I are not reliable
biomarkers for metronomic chemotherapy. |
References
1. Pramanik R, Bakhshi
S. Metronomic therapy in pediatric oncology: A snapshot.
Pediatr Blood Cancer. 2019;66: e27811.
2. Bahl A, Bakhshi S.
Metronomic chemotherapy in progressive pediatric
malignancies: Old drugs in new package. Indian J Pediatr.
2012;79:1617-22.
3. Motzer RJ,
Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA,
et al. Activity of SU11248, a multitargeted inhibitor of
vascular endothelial growth factor receptor and
platelet-derived growth factor receptor, in patients with
metastatic renal cell carcinoma. J Clin Oncol.
2006;24:16-24.
4. Rini BI, Michaelson
MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, et
al. Antitumor activity and biomarker analysis of
sunitinib in patients with bevacizumab-refractory metastatic
renal cell carcinoma. J Clin Oncol. 2008;26:3743-8.
5. Stempak D, Gammon J,
Halton J, Moghrabi A, Koren G, Baruchel S. A pilot
pharmacokinetic and antiangiogenic biomarker study of
celecoxib and low-dose metronomic vinblastine or
cyclophosphamide in pediatric recurrent solid tumors. J
Pediatr Hematol Oncol. 2006;28:720-8.
6. Kieran MW, Turner
CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, et al.
A feasibility trial of antiangiogenic (metronomic)
chemotherapy in pediatric patients with recurrent or
progressive cancer. J Pediatr Hematol Oncol. 2005;27:573-81.
7. Robison NJ,
Campigotto F, Chi SN, Manley PE, Turner CD, Zimmerman MA,
et al. A phase II trial of a multi-agent oral
antiangiogenic (metronomic) regimen in children with
recurrent or progressive cancer. Pediatr Blood Cancer.
2014;61:636-42.
8. Kesari S, Schiff D,
Doherty L, Gigas DC, Batchelor TT, Muzikansky A, et al.
Phase II study of metronomic chemo-therapy for recurrent
malignant gliomas in adults. Neuro-Oncol. 2007;9:354-63.
9. Pramanik R, Agarwala
S, Gupta YK, Thulkar S, Vishnubhatla S, Batra A, et al.
Metronomic chemotherapy vs best supportive care in
progressive pediatric solid malignant tumors: A randomized
clinical trial. JAMA Oncol. 2017;3:1222-7.
10. Willett CG, Boucher
Y, Duda DG, di Tomaso E, Munn LL, Tong RT, et al.
Surrogate markers for antiangiogenic therapy and
dose-limiting toxicities for bevacizumab with radiation and
chemotherapy: continued experience of a phase I trial in
rectal cancer patients. J Clin Oncol. 2005; 23:8136-9.
11. Burstein HJ, Chen
Y-H, Parker LM, Savoie J, Younger J, Kuter I, et al.
VEGF as a marker for outcome among advanced breast cancer
patients receiving anti-VEGF therapy with bevacizumab and
vinorelbine chemotherapy. Clin Cancer Res 2008;14:7871-7.
12. Tuszynski GP, Gasic TB, Rothman VL, Knudsen KA, Gasic GJ.
Thrombospondin, a potentiator of tumor cell metastasis.
Cancer Res. 1987;47:4130-3.

