|
|
|
Indian Pediatr 2019;56: 476-480 |
 |
Comparison of Feeding Options for HIV-Exposed
Infants: A Retrospective Cohort Study
|
|
Sandip Ray, Anju Seth, Noopur Baijal, Sarita Singh,
Garima Sharma, Praveen Kumar and Jagdish Chandra
From Department of Pediatrics, Lady Hardinge Medical
College, New Delhi, India
Correspondence to: Dr Anju Seth, Director Professor,
Department of Pediatrics, Lady Hardinge Medical College,
New Delhi, India.
Email: [email protected]
Received: September 10, 2018;
Initial review: December 27, 2018;
Accepted: March 20, 2019.
|
|
Objectives: To compare growth,
anemia prevalence, and sickness frequency in HIV- exposed uninfected
infants on different feeding modes. Methods: In this
retrospective cohort study, 109 HIV-exposed uninfected infants
registered at our center were categorized into three
groups as per their feeding mode during first 6 months viz.
exclusively breast fed (n=50), animal milk fed (n=40) and
commercial infant formula fed (n=19). Their anthropometric
parameters, hemoglobin and frequency of sickness at the age of 6 months
were compared. Results: There were no significant inter-group
differences in the weight for age, weight for length, length for age
z-scores (P=0.16, 0.37 and 0.12, respectively); proportion of
infants with underweight (P=0.63), wasting (P=0.82), or
stunting (P=0.82), and mean hemoglobin levels among the 3 groups
at 6 month of age. Animal milk fed and formula fed infant had increased
risk of sickness compared to exclusively breastfed infants (OR 2.5 and
2.49, respectively; P<0.01). Conclusions: In circumstances
where breastfeeding is not feasible or preferred, animal milk feeding
offers a viable alternative to commercial infant feeding formula in HIV
exposed infants.
Keywords: Animal milk, Breastfeeding, Infant
feeding, Formula-milk, Growth.
|
|
W
orld Health Organization (WHO) and National AIDS
Control Organisation (NACO) recommend exclusive breastfeeding (EBF) with
provision of lifelong antiretroviral treatment as the strategy of choice
to optimize HIV-free survival among HIV-exposed infants. In situations
where mothers cannot/choose not to breastfeed, the only option
recommended by WHO for infants <6 months is commercial infant formula
(CIF). Animal milk (AMF) is not recommended as a replacement feeding
option in the first six months of life [1]. However, the cost of CIF is
prohibitive in low- and middle-income countries and it may not be
economically feasible for mothers to use CIF exclusively for the first 6
months. In its recommendations, NACO has included locally available
unmodified animal milk apart from CIF as an option for replacement
feeding [2]. The former includes fresh boiled animal milk or pre-packed
processed toned dairy milk along with multi-vitamin and iron
supplementation. The Infant and Young Child Feeding (IYCF) Guidelines
(2010) of Indian Academy of Pediatrics also included unmodified animal
milk as an alternative option where EBF is not possible [3].
It is highly pertinent that AMF be formally evaluated
for its suitability as an alternative feeding option for HIV- exposed
infants. Otherwise, cost-cutting can compromise the infants’ growth and
development in the long run, an unacceptable consequence. This study
aimed to assess the impact of AMF on growth parameters, prevalence of
anemia and episodes of sickness in HIV-exposed infants as compared to
infants who received EBF or CIF during the first 6 months of life.
Methods
The present study is a retrospective analysis of
records of HIV-exposed infants registered at anti–retroviral therapy
center of Lady Hardinge Medical College and associated Kalawati Saran
Children’s Hospital at New Delhi during October 2007 to December 2015.
In accordance with the national policy, all
pregnant/lactating women with HIV infection and their infants registered
at our centre are given antiretroviral drugs (ARVs) for prevention of
HIV transmission to their infants [4]. They are counseled regarding
infant feeding options during pregnancy and again soon after birth by
trained counselors. In women opting for it, EBF is initiated within
first hour of birth. In situations where the woman opts for replacement
feeding or where EBF is not possible (maternal death or sickness),
feeding with locally available AMF (fresh boiled animal milk or
pre-packed processed toned dairy milk containing 3% fat, 3.1% protein
providing 58 kcal/100 mL) or CIF is decided upon after discussion with
the family depending upon their socio-economic condition and
socio-cultural factors [2]. Depending upon the opted feeding method, the
family is counseled regarding correct breastfeeding practices, avoidance
of mixed feeding and proper method of preparing and administering RF
using katori-spoon. Multi-vitamins to meet the RDA are started at
birth/time of registration at our centre and iron supplementation at the
rate of 2 mg/kg/day is prescribed at 6 weeks of age to all infants on
AMF. The compliance to feeding practices and supplements is ensured at
time of each contact by taking regular feedbacks and targeted
counseling.
For this study, we reviewed records of all
HIV-exposed infants registered at our ART centre within 15 days of birth
and followed up till at least 6 months of age. We excluded infants who
were lost to follow-up, who had less than two growth parameter reading
during the 6-month period, those who were given mixed feeding, and those
whose final HIV status was not determined at six months of age.
There was a change in National policy for prevention
of parent to child transmission of HIV (PPTCT) during this time. Prior
to January 2014, all women with HIV and their newborns were given a
single dose of nevirapine (NVP) during labour and immediately after
birth, respectively. After January 2014, all HIV-infected pregnant women
were initiated on ART soon after detection of their HIV status [4].
Infants born to these women were started on daily NVP prophylaxis at
birth and continued for a minimum of 6 weeks. Mothers who were detected
to be HIV-infected during labour/after delivery were started on ART upon
detection and their infants were given NVP prophylaxis. The study
therefore included data from participants registered before and after
change in recommendations. Determination of HIV status was done through
virological tests [4] i.e. HIV-1 DNA PCR by dried blood spot followed by
confirmation on whole blood if positive, at ages 6 weeks, 6 months and 6
weeks after stopping breastfeeding. The enrolled infants were classified
into three groups on basis of feeding strategy adopted during first 6
months following birth: (i) EBF: Infants exclusively breastfed; (ii)
AMF: infants receiving fresh animal milk (cow, buffalo or goat milk) or
commercially available pre-packed toned milk fed after boiling without
any modification; and (iii) CIF: infants receiving
age-appropriate commercially available infant feeding formula.
The nutritional status of infants was determined by
calculating z-scores for weight for age (WFA), weight for length (WFL)
and length for age (LFA) at birth and 6 months using WHO growth
reference standards [5], and prevalence of underweight (WFA <–2
z-score), wasting (WFL <–2 z-score) and stunting (LFA <–2 z-score) was
estimated. Episodes of sickness and serious sickness (requiring
hospitalization) till 6 months age, mean serum hemoglobin at 6 (±1)
months and grading of anemia severity as per WHO guidelines was also
assessed among the study infants [6]. The study protocol was approved by
the Institutional Ethics Committee.
Statistical analysis: Paired t-test was
used to compare the WFA, WFL and LFA z-scores of infants at birth and
six months in each feeding group. Analysis of co-variance (ANCOVA) was
used to compare the z-scores at six months among the three
feeding groups taking baseline z-score at birth as co-variant.
McNemar test was applied to compare the proportion of babies with
underweight, wasting and stunting at birth with that at six months.
Fisher Exact test was used to compare the proportion of babies with
underweight, wasting and stunting at six months among the three feeding
groups and for comparison of disease specific morbidities among the
three groups. For comparing the episodes of illness in infants among
different feeding groups, Poisson regression method was adopted. SPSS
version 23.0 (IBM, New York, USA) was used for statistical analyses.
Results
We evaluated case records of 189 infants (105 males)
for this study. At 6 months of age, 109 (57.7%) among these were
confirmed to be HIV-negative and 4 HIV-positive, while status of
remaining 76 infants was not determined. All the infants were
intra-mural referrals except 2 (4%) in EBF group. Baseline
characteristics of the 109 HIV-exposed uninfected infants included for
analysis are described in Table I. There was no mortality
among these infants during the first 6 months of age.
TABLE I Baseline Characteristics of Hiv-exposed Uninfected Infants (N=109)
|
Parameters, n (%) |
AMF (n=40) |
CIF (n=19) |
EBF(n=50) |
P value |
|
Vaginal delivery |
9 (22.5) |
7 (36.8) |
27 (54) |
|
|
Caesarean section |
31 (77.5) |
12 (63.2) |
23 (46) |
|
|
ART therapy in mothers* |
|
|
|
|
|
Started antenatally |
26 (65) |
13 (68.4) |
41 (82) |
|
|
Started perinatally |
12 (30) |
6 (31.6) |
6 (12) |
|
|
Not on ART at registration |
2 (5) |
0
|
3 (6) |
|
|
ARV prophylaxis status in infants |
|
|
|
|
|
Single dose nevirapine at birth |
19 (47.5) |
5 (26.3) |
14 (28) |
|
|
Nevirapine for 6-12 weeks after birth (with maternal ART) |
18 (45) |
12 (63.2) |
33 (66) |
|
|
Zidovudine till 12 wks age |
3 (7.5) |
2 (10.5) |
3 (6) |
|
|
Male infants |
25 (62.5) |
12 (63.1) |
29 (58) |
|
|
Gestational maturity, (n=30) |
|
|
|
|
|
Full-term |
11 (68.7) |
5 (83.3) |
6 (75) |
0.92 |
|
Preterm |
5 (31.3) |
1 (16.7) |
2 (25) |
0.47 |
|
Birthweight |
|
|
|
|
|
<2.5 kg (Low birth weight) |
11 (27.5) |
5 (20.3) |
15 (30) |
0.18 |
|
≥2.5 kg |
29 (72.5) |
14 (73.6) |
35 (70) |
0.95 |
|
Age at registration at ART center (d), mean (SD) |
3.1 (1.9) |
2.7 (1.7) |
2.3 (2.2) |
0.18 |
|
Anthropometric parameters at birth, mean (SD) |
|
|
|
|
|
Birthweight (kg) |
2.6 (0.5) |
2.7 (0.6) |
2.7 (0.4) |
0.68 |
|
WFA z-score
|
-1.3 (1.4) |
-1.3 (1.6) |
-1.3 (1.1) |
0.98 |
|
WFL z-score
|
-1.5 (1.3) |
-1.0 (1.6) |
-1.0 (1.3) |
0.21 |
|
LFA z-score
|
-0.7 (1.1) |
-0.4 (1.0) |
-1.0 (1.4) |
0.18 |
|
Underweight, n (%) |
8 (20) |
4 (21) |
13 (26) |
0.41 |
|
Washed, n (%)
|
11 (31.4) |
5 (29.4) |
9 (21.9) |
0.40 |
|
Stunted, n (%)
|
4 (10.8) |
0
|
8 (17.7) |
0.53 |
|
*ART consisting of: T (Tenofovir), L (Lamivudine), E (Efavirenz);
AMF: Animal milk; CIF: Commercial infant formula; EBF: Exclusive
breastfeeding. |
The anthropometric parameters in the three feeding
groups at 6 months, and mean change in z-scores at 6 months compared to
birth are shown in Table II. There was no difference in
LFA, WFA or WFL z-scores or in proportion of infants with underweight,
wasting or stunting at the age of six months among the three groups (Table
II).
TABLE II Comparison of Anthropometric Parameters at 6 Months of Age Among the Study Infants
|
Parameters |
AMF |
CIF |
EBF
|
P |
|
(n=40) |
(n=19) |
(n=50) |
value |
|
WFA z-score |
-1.5 (1.4) |
-0.7 (1.7) |
-1.4 (1.4) |
0.16 |
|
Change in z-score* |
-0.2 (1.8) |
0.5 (1.1) |
-0.1 (1.1) |
|
|
WFL z-score |
-0.7 (1.4) |
-0.2 (1.4) |
-0.7 (1.4) |
0.37 |
|
Change in z-score* |
0.8 (2.5) |
1 (1.4) |
0.5 (1.5) |
|
|
LFA z-score |
-1.4 (1.4) |
-0.8 (1.7) |
-1.4 (1.0) |
0.12 |
|
Change in z-score* |
-0.6 (1.3) |
-0.2 (1.4) |
-0.6 (0.9) |
|
|
Underweight (%) |
29.4 |
26.7 |
37.8 |
0.63 |
|
Wasting (%) |
16.7 |
7.1 |
18.9 |
0.82 |
|
Stunting (%) |
31.2 |
28.6 |
30 |
0.82 |
|
*as compared to birth z-score WFA; weight for age, WFL: weight
for length, LFA: length for age; AMF: fresh animal milk fed,
CIF: commercial infant formula fed, EBF: exclusively breast fed. |
Tables III shows frequency and nature of
illness among infants in the study groups. Two infants required
hospitalization during the study period, 1 each for sepsis (EBF group)
and diarrhea (AMF group). The AMF infants had higher odds (OR 2.5; 95%
CI: 1.4-4.4) of having an episode of illness compared to EBF group.
Likewise, CIF infants had higher odds (OR 2.49; 95% CI: 1.3-4.8) of
having an episode of illness compared to EBF group. There was no
significant difference between frequency of sickness among the AMF and
CIF groups.
TABLE III Comparison of Morbidity Profile Among the Study Infants During First 6 Months of Life
|
Illness type |
AMF
|
CIF |
EBF
|
|
(n=40) |
(n=19) |
(n=50) |
|
Total morbidities |
36 |
17 |
18 |
|
Number of episodes/child / 6 mo
|
0.9 |
0.9 |
0.4 |
|
Diarrhea* |
28 |
6 |
7 |
|
LRTI |
1 |
3 |
1 |
|
URTI |
6 |
3 |
5 |
|
Meningitis |
0 |
1 |
0 |
|
Skin (pyoderma/scabies) |
0 |
1 |
2 |
|
Sepsis |
0 |
2 |
1 |
|
Otitis media |
0 |
1 |
0 |
|
LRTI: lower respiratory tract infections;
URTI: upper respiratory tract infections; *significantly
different among the 3 study groups (P<0.001).
|
Hemoglobin levels at 6 months were available for 53
infants (AMF 16, CIF 9, EBF 28). Mean (SD) hemoglobin levels in infants
of AMF, CIF and EBF groups were 10.8 (1.41) g/dL, 11.0 (1.06) g/dL
and 10.2 (1.1) g/dL, respectively. There was no significant difference
in the mean hemoglobin level as well as proportion of infants with
different grades of anemia among the three groups (Fig. 1).
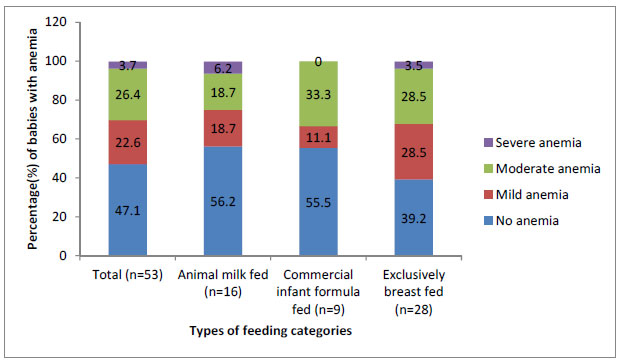 |
|
Fig. 1 Proportion of infants with
different grades of anemia in different feeding categories.
|
Discussion
TThis study documented no significant difference in
growth parameters or prevalence of anemia in HIV-exposed infants on AMF
as compared to CIF or EBF during first 6 months of life. Despite ongoing
feeding counseling, incidence of sickness, especially diarrhea, was
significantly higher in infants on AMF and CIF as compared to those on
EBF. There was no evidence of any significant difference between AMF and
CIF with respect to frequency of sickness.
The search for an option for replacement feeding that
would be culturally acceptable, economically viable while being
nutritionally adequate, is a felt need for HIV exposed infants [7]. AMF
has been widely used for infant feeding by women with HIV infection
[8,9]. Papathakis, et al. [10] have shown home prepared
modifications of evaporated milk, powdered full cream milk and fresh
full cream milk to be deficient in vitamins and essential minerals. We
could not find any published work which has reported on growth
parameters or anemia prevalence among infants (HIV-exposed or otherwise)
fed on animal milk. While no difference has been reported in
anthropometric parameters of HIV-exposed infants on EBF or CIF from
South Africa [11], higher weight for height z-scores over a two year
follow-up have been reported in breast fed as compared to formula fed
infants from Kenya [12]. No difference in anemia prevalence has been
reported in infants fed on EBF and CIF [11,13]. An increased risk of
morbidity and hospitalization in HIV-exposed infants on replacement
feeding, compared to those on EBF, has been shown in a previous Indian
study [9]. Studies from other parts of the world have also shown higher
frequency of respiratory tract infections [14] and diarrhea [11,12,14]
in non-breastfed infants. WHO has published a meta-analysis showing
breastfeeding to be protective against deaths due to infectious
diseases, the odds being significantly more in first six months of life
than in later ages [15].
Despite widespread use of animal milk in infant
feeding, especially in context of maternal HIV infection, the evidence
for suitability of animal milk as an option for replacement feeding in
HIV-exposed infants is lacking. Retrospective nature of data is a major
limitation of this study. While we have demonstrated that use of AMF
along with multi-vitamin and iron supplementation in HIV- exposed
infants leads to an equivalent growth, anemia prevalence and similar
morbidity outcomes as compared to CIF, we have not been able to assess
the status of other micro-nutrients in these infants. We have also not
measured serum ferritin levels, a better marker for iron deficiency.
This work provides data that AMF along with iron and
multivitamin supplementation is a possible alternative to CIF in
HIV-exposed infants where breastfeeding is not feasible/opted for.
However, there is a need to standardize the nutrient value of these milk
preparations as well as study the micronutrient status of the infants
fed exclusively on AMF, so that holistic growth and development of an
infant does not suffer.
Contributors: SR: extracted the data, analyzed it
and drafted the paper; AS: conceptualized the paper, was overall
responsible for quality of data collection and maintenance, modified &
finalized the draft; NB, SS, GS: clinical care, and data recording and
analysis; JC, PK: patient care, quality maintenance and modification of
draft of the paper. All authors provided inputs to manuscript writing,
and its final approval.
Funding: None; Competing
interest: None stated.
|
What This Study Adds?
• Animal milk feeding, along with iron and
multivitamin supplementation, offers a viable alternative to
commercial infant feeding formula in terms of anthropometric and
morbidity-related outcomes in HIV-exposed infants.
|
References
1. World Health Organization, United Nations
Children’s Fund. Guideline: updates on HIV and Infant Feeding: The
Duration of Breastfeeding, and Support from Health Ser-vices to Improve
Feeding Practices Among Mothers Living With HIV. Geneva: World Health
Organization; 2016./p>
2. National AIDS Control Organization. Nutrition
Guidelines for HIV-Exposed and Infected Children (0-14 Years of Age).
Available from:
http://naco.gov.in/sites/default/files/Paedia%20Nutrition%20national%20guidelines%20
NACO.pdf. Accessed January 07, 2013.
3. Rajeshwari K, Bang A, Chaturvedi P, Kumar V, Yadav
B, Bharadva K, et al. Infant and Young Child Feeding Guidelines:
2010. Indian Pediatr. 2010;47:995-1004.
4. National AIDS Control Organization-Updated
Guidelines for Prevention of Parent to Child Transmission (PPTCT) of HIV
Using Multi-drug Anti-retroviral Regimen in India, December 2013.
Available from: i>
http://naco.gov.in/sites/default/files/National_Guidelines_for_PPTCT_0.pdf.
Accessed April 04, 2018.
5. World Health Organization. WHO Child Growth
Standards. 2006. Available from: http://www.who.int/child
growth/standards/Technical_report.pdf. Accessed 04 April, 2018.
6. World Health Organization. Hemoglobin
Concentrations for the Diagnosis of Anemia and Assessment of Severity:
WHO/NMH/NHD/MNM/11.1. Available from:
http://www.who.int/vmnis/indicators/haemoglobin.pdf.. Accessed April
04, 2018.
7. Cames C, Mouquet-Rivier C, Traore T, Ayassou KA,
Kabore C, Bruyeron O, et al. A sustainable food support for
non-breastfed infants: implementation and acceptability within a WHO
mother-to-child HIV transmission prevention trial in Burkina Faso.
Public Health Nutr. 2010;13:779-86.
8. Van Hollen C. Breast or bottle? HIV-positive
women’s responses to global health policy on infant feeding in India.
Med Anthropol Q. 2011;25:499-518.
9. Phadke MA, Gadgil B, Bharucha KE, Shrotri AN,
Sastry J, Gupte NA, et al. Replacement-fed infants born to
HIV-infected mothers in India have a high early postpartum rate of
hospitalization. J Nutr. 2003;133:3153-7.
10. Papathakis PC, Rollins NC. Are WHO/UNAIDS/UNICEF
recommended replacement milks for infants of HIV-infected mothers
appropriate in the South African context? Bull World Health Organ.
2004;82:164-71.
11. Kindra G, Coutsoudis A, Esposito F, Esterhuizen
T. Breastfeeding in HIV exposed infants significantly improves child
health: A prospective study. Matern Child Health J.i>
2012;16:632-40.
12. Mbori-Ngacha D, Nduati R, John G, Reilly M,
Richardson B, Mwatha A, et al. Morbidity and mortality in
breastfed and formula-fed infants of HIV-1- infected women: A randomized
clinical trial. JAMA. 2001;286:2413-20.
13. Nduati R, John G, Mbori-Ngacha D, Richardson B,
Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula
feeding on transmission of HIV-1: A randomized clinical trial. JAMA.
2000;283:1167-74.
14. Becquet R, Bequet L, Ekouevi DK, Viho I,
Sakarovitch C, Fassinou P, et al. Two-year morbidity-mortality
and alternatives to prolonged breast-feeding among children born to
HIV-infected mothers in Cote d’Ivoire. PLoS Med. 2007;4:e17.
15. WHO Collaborative Study Team on the Role of
Breastfeeding on the Prevention of Infant Mortality. Effect of
breastfeeding on infant and child mortality due to infectious diseases
in less developed countries: A pooled analysis. Lancet. 2000;355:451-5.
|
|
|
 |
|

