|
|
|
Indian Pediatr 2019;56: 472-475 |
 |
Association Between Neonatal Thyroid
Stimulating Hormone Status and Maternal Urinary Iodine Status
|
|
Haseena Sait 1,
Seema Kapoor1,
Ankur Jindal1,
Ritika Garg1,
Ravi Shankar Belwal3,
Sangita Yadav1,
Sangeeta Gupta2
and BK Thelma4
From Departments of 1Pediarics, and 2Obstretics
and Gynecology, Lok Nayak Hospital and Maulana Azad Medical College;
3Human Nutrition Unit, AIIMS; and 4Department of Genetics, University of
Delhi – South Campus; New Delhi, India.
Correspondence to: Dr Seema Kapoor, Director
Professor, Division of Genetics and Metabolism, Department of
Pediatrics, Lok Nayak Hospital and Maulana Azad Medical College, New
Delhi, India.
Email: [email protected]
Received: July 14, 2018;
Initial review: October 03, 2018;
Accepted: April 06, 2019.
|
|
Background: Maternal urinary
iodine concentration (MUIC) and percentage of neonates with Thyroid
stimulating hormone (TSH) >5 mIU/L are amongst the parameters suggested
for assessing adequate iodine status.
Objective: To assess the
correlation between MUIC and neonatal TSH levels.
Study design: Cross-sectional.
Settings: Tertiary care center in
Delhi, India, between November 2015 to November 2016.
Participants: Postnatal
mother-neonate dyads.
Methods: TSH levels assessed
among neonatal samples were stratified as below and above 5 mIU/L. MUIC
was measured in 544 mothers, 400 mother-neonate dyads with neonatal TSH
levels >5 mIU/L (cases) and 144 mother-neonate newborn mother dyads with
neonatal TSH <5 mIU/L (controls).
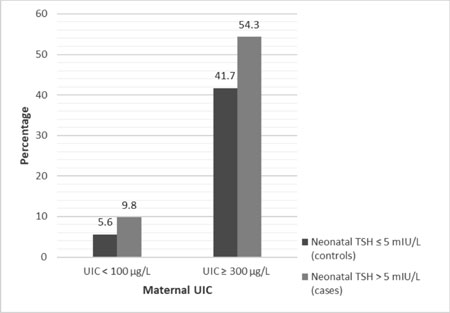
Results: The percentage of
mothers with iodine insufficiency (9.8% vs 5.6%) as well as
iodine excess (54.3% vs 41.7%) were significant higher in cases
than controls. Mean TSH was also higher (P=0.0002) in both the
iodine deficient and iodine excess group. There was no correlation
between neonatal TSH values and MUIC.
Conclusions: Lack of correlation
between neonatal TSH and MUIC is due to iodine excess together with
iodine deficiency.
Keywords: Newborn screening, Postpartum,
Povidone iodine, Pregnant, TSH.
|
|
I
odine is crucial for the production of thyroid
hormones and hence an important determinant of maternal and neonatal
health. Pregnant women and newborn children are most vulnerable to
Iodine deficiency disorders (IDD) [1]. As per World Health Organization
(WHO), iodine status of a population can be assessed by parameters such
as Total Goiter Rate (TGR), Maternal urinary iodine concentration
(MUIC), thyroglobulin levels in school-aged children, and neonatal
thyroid stimulating hormone (TSH) levels. The neonatal thyroid has a low
iodine content compared to the adult thyroid, and hence neonatal iodine
turnover is much higher, especially in case of iodine deficiency.
Consequently it has been assumed that the neonatal thyroid is extremely
sensitive to iodine deficiency [2]. Assessment of iodine status in a
population using MUIC and neonatal TSH concentrations is considered
complementary [3]. However, the correlation between the two has been
sparsely evaluated in the Indian context.
Recent estimates from surveys indicated that the
median UIC (urinary iodine concentration) of the Indian population was
154 µg/L [4]. While iodine deficiency still persists in many parts of
the country, iodine excess has recently been documented in a few studies
from Delhi [5,6]. Thus, we aimed at assessing the correlation, if any,
between MUIC and neonatal TSH levels, and to study the current iodine
status in a group of mother-infant dyads.
Methods
This study was performed in a tertiary care center in
New Delhi, India between November 2015 and November 2016. The study
protocol was approved by Institutional Ethics Committee. Term healthy
neonates weighing >2500 g and their mothers were included. Mothers who
were hypothyroid, had autoimmune disease (diagnosed as anti TPO antibody
positive), on antithyroid medication, requiring intensive postpartum
care or had blood stained urine were excluded. The details of the age of
mothers, parity, the type of salt used in their household were collected
by verbal information. The antiseptic used during delivery was recorded.
Neonatal TSH levels were assessed using Dried blood
spot (DBS) collected by heel prick method from newborns between 24-48
hours after birth, as a part of an ongoing newborn screening project
(NBS). It was ensured that the neonates were not exposed directly to
povidone iodine. Neonatal TSH values >10 mIU/L (whole blood units) were
excluded as it fell into the ambiguous zone. Simultaneously spot urine
samples were collected from their mothers after obtaining informed and
written consent. Spot urine samples of mothers processed in the
postpartum period (Day 1-Day 7) whose neonates had TSH values >5 mIU/L
were considered as case group, whereas those from postpartum mothers
whose neonates had TSH values £5
mIU/L were considered as control group. Spot urine samples from 114
healthy pregnant mothers were also collected at term gestation. This
group was considered after the study was initiated as the initial
results were contrary to those envisaged in the original hypothesis, and
to check whether the results of the MUIC could be related to use of
povidone iodine during delivery.
Cut-offs and recommendations of the procedures to be
followed were adopted from guidelines released by WHO [7]. According to
WHO, the proportion of neonates with TSH values >5 mIU/L in whole blood
is proportional to the degree of iodine deficiency during pregnancy.
When a sensitive TSH assay is performed on the samples, <3% frequency of
TSH values >5 mIU/L indicates iodine sufficiency in a population.
The cut-off values to define a population having
iodine sufficiency are median urinary iodine concentration between
100-199 µg/L in children, 150-249 µg/L in pregnant women and
³100µg/L in lactating
women. Iodine insufficiency in postpartum and pregnant mothers was
considered at urinary iodine levels <100 µg/L and <150 µg/L,
respectively; iodine levels between 250-499 µg/L were considered as
‘above requirements’ in pregnant mothers; and iodine excess was
considered at levels ³300
µg/L and ³500
µg/L during postpartum and pregnancy period, respectively.
TSH was measured by time resolved fluro-immunoassay
on the Genomic screening processor (Perkin Elmer Life Sciences, Turku
Finland). The limit of detection of the assay was 2 µU/L and the
coefficient of variation <5%. The iodine concentration in urine was
measured by the Wet Digestion method with internal quality control, and
the results were expressed as µg/L.
The sample size of 400 mother neonatal dyads was
estimated with the power of 90% and alpha error of 5%, based on a
previous study which established the correlation between neonatal TSH
and MUIC (r = -0.67) [8].
Statistical analysis: The analysis was done using
STATA 11 software. Chi-square test was used to identify the relationship
between neonatal TSH status ( £
5 or >5 mIU/L) and MUIC. One way ANOVA with post-hoc Tukey HSD (Honest
significant difference) test was used to compare TSH levels in iodine
deficient, sufficient and excess group based on urinary iodine levels.
The Spearman rank test was used to identify the correlation between MUIC
and neonatal TSH levels.
Results
The total number of deliveries in our institution
during the study period was 12785. The number of neonates having TSH
between 5.1-10 mIU/L was 1555 (12.1%). After the maternal and neonatal
exclusion factors, 1050 neonates were found eligible. Out of these,
urinary samples from a convenient sample of 400 mothers of cases and 144
mothers of controls were collected.
The general characteristics of the mothers and the
neonates in the two groups are detailed in Table I.
Iodized salt consumption was noted in 97% of the population. Povidone
iodine (5%) was used as an antiseptic for conducting deliveries in all
the patients. The mean maternal urinary iodine levels were 223 µg/L and
240 µg/L in cases and controls, respectively. The proportion of mothers
with iodine insufficiency as well as iodine excess were significantly
higher in cases than controls (Table I and Fig.
1). When mothers were grouped based on urinary iodine levels as
iodine deficient, sufficient and excess, it was found that mothers
excreting insufficient (<100 µg/L) and excess iodine ( ³300
µg/L) had significantly increased neonatal TSH levels than iodine
sufficient group (P<0.001). It was also found that iodine
excretion in urine was significantly lower in mothers who consumed
non-iodized salt (P=0.03). The mode of delivery had a significant
association (P <0.001) with MUIC, with levels being higher in
mothers who underwent caesarean section. There was no significant
correlation observed between neonatal TSH levels and MUIC.
TABLE I Comparison of Parameters between Cases and Controls (N=544)
|
Neonatal TSH Levels
|
|
≤ 5 mIU/L
|
>5mIU/L
|
|
(n=144) |
(n=400) |
|
Maternal age (y)* |
26.3 (4.5) |
25.8 (3.9) |
|
Primipara#
|
49 (34) |
176 (44) |
|
Caesarian delivery# |
38 (26.4) |
98 (24.5) |
|
Maternal UIC (µg/L)* |
223 (78.3) |
240 (103.5) |
|
Male gender;n (%) |
74 (51.3) |
221 (55.3) |
|
Birth weight (g)* |
2818 (475) |
2921 (314) |
|
Maternal UIC <100 µg/L# |
8 (5.5) |
40 (9.8) |
|
Maternal UIC ≥300 µg/L# |
60 (41.7) |
217 (54.3) |
|
Cases=TSH >5 mIU/L; Controls = TSH <5 mIU/L; UIC: urinary
iodine concentration; *Mean (SD); #n (%). |
 |
|
Fig. 1 Maternal iodine deficiency and
excess in the enrolled neonates.
|
Among the pregnant mothers, the mean urinary iodine
level was 242.8 (74.8) µg/L. Iodine insufficiency (UIC <150 µg/L) was
present in 13.6% whereas more than adequate urinary iodine (UIC
³250 µg/L) was
present in 65.3%.
Discussion
In this cross-sectional hospital-based study, we
observed that maternal iodine insufficiency as well as excess was more
frequent among neonates with TSH >5 mIU/L than those with level
£5 mIU/mL. Mean
neonatal TSH was also higher in those postpartum mothers who excreted
insufficient or excess iodine. There was no correlation observed between
MUIC and neonatal TSH. 65.3% pregnant mothers had more than adequate
iodine in their urine; insufficiency was observed in only 13.6% of the
pregnant mothers.
The finding of iodine excess in our study is in
consonance with the data from school-going children of upper
socioeconomic strata [6], which demonstrated that 83% of the children
had urinary iodine concentration (UIC)
³300 µg/L. Grewal,
et al. [5], also reported trimester specific UIC of 150 pregnant
women to be in the range of 304 µg/L, and 77.4% had UIC
³250 µg/L. Both the
groups attributed the finding to improvement in the implementation of
universal salt iodization and additionally other non-salt sources of
iodine.
According to WHO, the proportion of neonates with TSH
values >5 mIU/L in whole blood is proportional to the degree of iodine
deficiency during pregnancy. Elevated neonatal serum TSH concentration
may indicate insufficient supply of thyroid hormones to the developing
fetal brain, and is therefore the only measure that allows prediction of
brain damage due to iodine deficiency [2]. The cutoff of neonatal TSH >5
mIU/L used in our study has been suggested to be a good discriminator
for severe iodine deficiency in the population; though, reports from
certain areas of mild deficiency suggest it to be questionable.
The reasons for elevation of TSH in iodine deficiency
are implicit. However, elevation of neonatal TSH levels in iodine excess
group is contrary to the popular belief; however, it could be explained
by a mechanism called Wolff-Chaikoff effect [9]. Both iodine excess and
deficiency causing a raised neonatal TSH level might have resulted in a
lack of correlation between MUIC and neonatal TSH levels.
The main strength of this study was the
identification of recent tilt in the axis of iodine status in females,
and the same finding even in pregnant mothers who were not exposed to
povidone iodine. This emphasizes the fact that closer surveillance on
both salt and non-salt sources of iodine should be done. Its long term
implication has not been evaluated in terms of neonatal cognitive domain
and general health. Extrapolation from adult data from India [10,11]
suggests increasing autoimmunity and anti-TPO antibody positivity, which
may have an impact on long-term neonatal health.
There were few limitations of this study. First, the
collection of neonatal blood samples between 24-48 h may not be the best
strategy for evaluating the TSH status. The proportion of population
consuming iodized salt was recorded but we did not measure the content
of iodine in salt samples on the basis of recall. Re-estimation of
urinary iodine beyond the postpartum period or analysis of other thyroid
parameters like thyroglobulin was also not performed in this study. As
iodine is also excreted in breast milk, MUIC may lead to underestimation
of iodine intake.
We conclude that neonatal TSH levels have no direct
relationship with maternal urinary iodine levels; iodine insufficiency
as well as excess seem to be higher among mothers of newborns with high
(>5 mIU/L) TSH levels at 24-48 hours. Though iodine deficiency was still
present in a proportion of the study samples, the looming burden of the
excess is indeed a matter of concern. Few countries [12,13] have already
entered the post-iodization era and report iodine excess. Further large
scale multicentric studies across geographical zones, including coastal
and mainland areas, are required along with robust parameters of
assessment such as thyroglobulin and iodine content of the salt,
relationship between maternal iodine and neonatal thyroid status.
Contributors: HS, SK, BKT: conceived the idea of
the project; SY,RG: were involved in management; AJ, RB: was responsible
for the laboratory an alysis; SG: managed the mothers antenatally; All
the authors have contributed to manuscript writing and approved the
final version.
Funding: Science and Engineering Research Board,
New Delhi for NBS study in Delhi state vide Grant # IR/SO/LC-0001/2012.
Competing interest: None stated.
|
What is Already Known?
• The iodine status of the nation is not yet
sufficient and intensive universal salt iodization programs are
in place to address this issue.
What This Study Adds?
• The proportion of mothers with iodine
insufficiency as well as excess is more in newborns with
deficient thyroid status (TSH >5 mIU/L).
|
References
1. Pandav CS, Yadav K, Srivastava R, Pandav R,
Karmarkar MG. Iodine deficiency disorders (IDD) control in India. Indian
J Med Res. 2013;138:418-33.
2. Vandevijvere S, Coucke W, Vanderpas J, Trumpff C, Fauvart
M, Meulemans A, et al. Neonatal thyroid-stimulating hormone
concentrations in Belgium: A useful indicator for detecting mild iodine
deficiency? PLoS One. 2012;7:e47770.
3. Vanderpump MPJ, Lazarus JH, Smyth PP, Laurberg P,
Holder RL, Boelaert K, et al. Iodine status of UK schoolgirls: A
cross-sectional survey. Lancet. 2011;377:2007-12.
4. Rah JH, Anas AM, Chakrabarty A, Sankar R, Pandav
CS, Aguayo VM. Towards universal salt iodisation in India: Achievements,
challenges and future actions. Matern Child Nutr. 2015;11:483-96.
5. Grewal E, Khadgawat R, Gupta N, Desai A, Tandon N.
Assessment of iodine nutrition in pregnant north Indian subjects in
three trimesters. Indian J Endocrinol Metab. 2013;17:289-93.
6. Marwaha RK, Tandon N, Desai A, Kanwar R, Mani K.
Iodine nutrition in upper socioeconomic school children of Delhi. Indian
Pediatr. 2010;47:335-8.
7. World Health Organization. A Guide for Programme
Managers. Assessment of Iodine Deficiency Disorders and Monitoring their
Elimination. Geneva: World Health Organization; 2007.
8. Hamza R, Youssef A, Mouharam W, Danasoury AEl.
Maternal and neonatal iodine nutrition in Cairo. Internet J Pediatr
Neonatol. 2007;8:2.
9. Wolff J, Chaikoff IL. Plasma inorganic iodide as a
homeostatic regulator of thyroid function. J Biol Chem. 1948;174:555-64.
10. Usha Menon V, Sundaram KR, Unnikrishnan AG,
Jayakumar RV, Kumar NV. High prevalence of undetected thyroid disorders
in an iodine sufficient adult south Indian population. J Indian Med
Assoc. 2009;107:72.
11. Marwaha RK, Tandon N, Karak AK, Gupta N, Verma K,
Kochupillai N. Hashimoto’s thyroiditis: Countrywide screening of
goitrous healthy young girls in postiodization phase in India. J Clin
Endocrinol Metab. 2000;85: 3798-802.
12. Katagiri R, Yuan X, Kobayashi S, Sasaki S. Effect
of excess iodine intake onthyroid diseases in different populations: A
systematic review and meta-analyses including observational studies.
PLoS One. 2017;12:p.e0173722.
13. Bürgi H. Iodine excess. Best Pract Res Endocrinol Metab.
2010;24:107-15.
|
|
|
 |
|

