|
|
|
Indian Pediatr 2019;56:463-467 |
 |
Prophylactic Vitamin K
Administration in Neonates on Prolonged Antibiotic Therapy: A
Randomized Controlled Trial
|
|
Amanpreet Sethi 1,
M Jeeva Sankar1,
Anu Thukral1,
Renu Saxena2,
Suman Chaurasia1
and Ramesh Agarwal1
From Departments of 1Pediatrics and 2Hematology,
All India Institute of Medical Sciences, New Delhi, India.
Correspondence to: Dr Ramesh Agarwal, Department of Pediatrics, All
India Institute of Medical Sciences,
New Delhi 110 029, India.
Email: [email protected]
Received: May 29, 2018;
Initial review: October 15, 2018;
Accepted: April 16, 2019.
Trial registration: Clinical Trial Registry of India
(CTRI/2017/02/007776).
|
Objective: To compare the prevalence of vitamin K
deficiency after intramuscular vitamin K or no treatment in neonates
with sepsis on prolonged (>7 days) antibiotic therapy.
Study Design: Open label randomized controlled
trial.
Setting: Level 3 Neonatal Intensive Care Unit
(NICU).
Participants: Neonates with first episode of
sepsis on antibiotics for ³7
days were included. Neonates with clinical bleeding, vitamin K prior to
start of antibiotic therapy (except the birth dose), cholestasis or
prenatally diagnosed bleeding disorder were excluded.
Intervention: Randomized to receive 1 mg vitamin
K (n=41) or no vitamin K (n=39) on the 7th day of
antibiotic therapy.
Main outcome measure: Vitamin K deficiency
defined as Protein Induced by Vitamin K Absence (PIVKA-II) >2 ng/mL after
7 ± 2 days of enrolment.
Results: The prevalence of vitamin K deficiency
was 100% (n=80) at enrolment and it remained 100% even after 7 ±
2 days of enrolment in both the groups.
Conclusion: Neonates receiving prolonged
antibiotics have universal biochemical vitamin K deficiency despite
vitamin K administration on 7th day of antibiotic therapy.
Keywords: Antibiotics, Neonatal sepsis, PIVKA.
|
|
P
rolonged antibiotic therapy can lead to
deficiency of vitamin K either by eradication of gut flora, a common
source of mena-quinones or by direct inhibition of the vitamin K
dependent step in clotting factor synthesis by some antibiotics
containing 1-N-methyl-5-thiotetrazole (NMTT) side group [1]. Vitamin K
deficiency is a frequent complication in patients admitted in adult
intensive care units (ICU) with incidence as high as 25% [2]. One of the
important implicated risk factors is prolonged antibiotic usage, a
common scenario in neonatal intensive care units (NICU).
Prolonged antibiotic usage has been found to result
in vitamin K-related coagulopathy in about one-third of children with
higher incidence in malnourished children and infants [3]. Extrapolating
the evidence from adult and limited pediatric studies, most of the NICUs
in India prefer to administer vitamin K every 7 days to neonates on
antibiotic therapy. However, evidence for this practice is lacking in
the neonatal population. Studies on preterm neonates and neonates in
surgical ICU on prolonged antibiotic therapy have demonstrated high
levels of vitamin K after its single parenteral birth dose [4,5].
Therefore, this study was planned to revisit the practice of
administering vitamin K during prolonged antibiotic therapy amongst
neonates.
Methods
This open-label randomized clinical trial was
conducted in a level-3 neonatal unit in Northern India, between July
2015 and August 2016. Neonates with first episode of symptomatic sepsis
on antibiotics for 7 or more days were included. Neonates who received
vitamin K prior to start of antibiotic therapy (except the birth dose),
who had any clinical bleeding, cholestasis or prenatally diagnosed
bleeding disorder were excluded. Eligible neonates were enrolled on the
seventh day of antibiotic therapy after obtaining written informed
consent from the parents. The study was approved by the Institute Ethics
Committee, All India Institute of Medical Sciences, New Delhi, India.
Enrolled neonates were randomized at seventh day of
antibiotic therapy to receive single dose of 1 mg intramuscular vitamin
K or no vitamin K. Both vitamin K1 (Inj. Kenadione; Samarth Pharma
Private Ltd., Mumbai) and K3 (Inj. Reokay; Rathi Laboratories Private
Ltd, Patna) were used in the study as per their availability in the
central drug store of the hospital. All neonates received intramuscular
Vitamin K1 at birth as per the unit protocol (Weight
³1000 g: 1 mg and
<1000 g: 0.5 mg). Computer generated random numbers with variable block
size (2 to 8) were used to allot the neonates into the two groups. The
randomization sequence was prepared by another investigator who was not
involved in collecting baseline variables, applying the intervention and
measuring the outcomes. To ensure allocation concealment, random
treatment assignment was placed in serially numbered opaque and sealed
envelopes. Blinding of the intervention was not possible because of the
nature of the intervention. Vitamin K dose was administered by the nurse
on duty who was not involved in group allocation.
Primary outcome was the proportion of neonates with
PIVKA-II levels more than 2 ng/mL after 7±2 days of enrolment. Secondary
outcome was the proportion of neonates with coagulation abnormalities (prothrombin
time (PT): 2 seconds more than upper limit as per gestational age and
post-natal age cut-off [6]) after 7±2 days of enrolment.
At enrolment, 1 mL of blood sample was drawn by
venepuncture from all the neonates and sent for PIVKA II estimation in
the coagulation laboratory of the institute. Second sample was taken
after 7±2 days of enrolment. All the samples were centrifuged at 2500
rpm for 10 minutes and supernatant serum was decanted and stored at -70 ºC.
All the samples were analyzed together. For PIVKA II estimation, samples
were thawed in the water bath at 37ºC for 15 minutes. PIVKA-II
estimation was done using specialized kits with sandwich ELISA technique
(Flarebio Biotech, USA & Elabscience Biotechnology, China) and PT
estimation was done using an automated ACL Elite Pro system
(Instrumentation Laboratory, USA). During analysis, all the serum
samples tested above the highest detectable range of the kit (10 ng/mL).
So, standardization of the ELISA assay took extra efforts. In the next
step, six of the randomly selected samples were run in serial dilutions.
Eventually, at 1:128 dilution of the serum with buffer, the PIVKA-II
levels were in the detectable range. We used healthy adult blood samples
as control.
There was no published study that had used PIVKA-II
levels as surrogate marker for vitamin K deficiency in neonates on
prolonged antibiotics. So, sample size calculation was based on a study
in patients aged 3 months to 23 years, suffering from cystic fibrosis
and on prolonged antibiotic therapy where abnormal coagulation and
raised PIVKA-II levels were found in 33% of cases [7]. Thus, a total of
94 neonates (47 in each group) were required to have 80% chance of
detecting with significance at 5% level, a decrease in vitamin K
deficiency from 33% in the control group to 10% in the intervention
group.
Statistical analysis: Statistical analysis was
performed using Stata 11.2 (Stata-Corp, College station, Texas, US).
Categorical variables were compared by Chi square/Fisher Exact test.
Continuous variables were compared by Student t-test (if normally
distributed) or Wilcoxson rank sum Test (if skewed).
Results
A total of 80 neonates were enrolled out of 120
neonates with sepsis during the study period. Among enrolled neonates,
41 were allocated to vitamin K group and 39 were allocated to ‘no
vitamin K’ group (Fig. 1). Two neonates in the vitamin K
group died before outcome assessment because of severe sepsis. In three
neonates (2 in vitamin K group and 1 in no vitamin K group), second
blood sample could not be collected after 7±2 days of enrolment. Thus,
37 neonates were analyzed in the vitamin K group and 38 in ‘no vitamin
K’ group. Baseline demographic variables and postnatal morbidities were
comparable between the two groups (Table I).
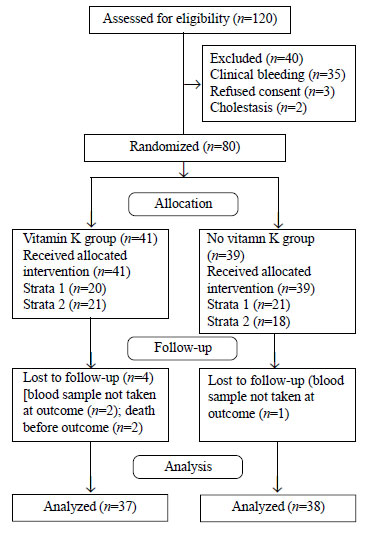 |
|
Fig. 1 Trial Flow.
|
TABLE I Baseline Demographic Variables and Postnatal Morbidities
|
Variable |
Vitamin K group |
No Vitamin |
|
(n=41) (%) |
K group |
|
|
(n=39) (%) |
|
Gestation (wks)* |
31.4 (4.0) |
32.5 (4.1) |
|
Birth weight (g)# |
1380 |
1394 |
|
(961-2005) |
(955-2043) |
|
Appropriate for gestational age |
29 (70.7) |
26 (66.6) |
|
Male |
19 (46.3) |
23 (58.9) |
|
Preterm premature rupture of membrane (> 24 h) |
7 (17) |
6 (15.3) |
|
Mother on drugs causing vitamin K deficiency |
3 (7.3) |
2 (5.1) |
|
Intrapartum antibiotics |
7 (17) |
7 (17.9) |
|
Clinical chorioamnionitis |
2 (4.8) |
0 |
|
Apgar at 5 min# |
8 (7-9) |
8 (7-9) |
|
Birth trauma |
1 (2.4) |
1 (2.5) |
|
Prophylactic vitamin K at birth |
41 (100) |
39 (100) |
|
Exclusive breast milk at enrolment |
34 (82.9) |
36 (92.3) |
|
Respiratory distress syndrome |
19 (46.3) |
11(28.2) |
|
Hyperbilirubinemia requiring phototherapy |
13(31.8) |
7 (18) |
|
Intraventricular hemorrhage |
4 (9.7) |
5 (12.8) |
|
Early onset sepsis (d” 72 h) |
24 (58.5) |
16 (41.0) |
|
Post-natal age at enrolment # (d) |
10.5 (9 to 18.2) |
10 (9 to 18) |
|
Blood culture positive sepsis |
3 (7.3) |
5 (12.8) |
|
Hypotension requiring inotropes |
11(26.8) |
9 (23.1) |
|
Total parenteral nutrition (TPN) at enrolment |
4 (9.7) |
2 (5.1) |
|
Duration of antibiotics during the sepsis episode* (d)
|
10.8 (5.4) |
12.1 (6.3) |
|
Values in n (%) except *Mean (SD) and #Median (IQR). |
The baseline prevalence of vitamin K deficiency was
100% in both the groups. Quantitative PIVKA-II levels could be done in
only 37 neonates (20 in the vitamin K group and 17 in the no vitamin K
group). Mean (SD) PIVKA-II levels were very high in both the groups with
no significant difference between the two groups (Table II).
TABLE II Prevalence of Vitamin K Deficiency and PIVKA II Levels at Enrolment
|
Variable |
Vitamin K group |
Control group |
|
PIVKA-II >2 ng/mL, n (%) |
(n=41) 41 (100) |
(n=37) 37 (100) |
|
PIVKA-II levels, mean (SD) |
(n= 20) 992 (215) |
(n= 17) 929 (263) |
The prevalence of vitamin K deficiency after 7±2 days
of enrolment was 100% in both the groups. Quantitative PIVKA-II levels
could be done in only 35 neonates (18 in the vitamin K group and 17 in
the no vitamin K group). Mean (SD) PIVKA-II levels were comparable
between the two groups (Table III). There was no
difference in the prevalence of coagulation abnormalities as assessed by
deranged PT or clinical bleeding between the two groups.
TABLE III Prevalence of Vitamin K Deficiency and PIVKA II Levels After 7 ± 2 Days of Enrolment
|
Variable |
Vitamin K group |
Control group |
Relative risk/Mean difference |
P value |
|
|
|
(95% CI) |
|
|
PIVKA-II >2 ng/mL, n (%) |
(n=37)37 (100) |
(n=38)38 (100) |
– |
1.00 |
|
PIVKA-II levels, mean (SD) |
(n= 18)946 (153)* |
(n= 17)959 (280) * |
-13 |
(-167 to 140) 0.86 |
|
Deranged PT, n (%) |
(n=31)3 (9.6) |
(n=34)1 (2.9) |
1.63(0.8 to 3.1) |
0.27 |
|
Clinical bleeding, n (%) |
(n=41)4(9.6) |
(n=39)1 (2.6) |
1.62(0.9 to 2.6) |
0.11 |
Discussion
This open label randomized controlled trial, compared
the effect of 1 mg parenteral vitamin K administration on seventh day of
antibiotic therapy versus no vitamin K on the prevalence of
vitamin K deficiency in neonates with sepsis. All neonates in both
groups were deficient in vitamin K when assessed for outcome.
The strength of our study is that PIVKA-II levels
were assessed by an independent clinician, blinded to the group
allocation, thus minimizing bias. The major limitation of our study was
that only 80 neonates out of the total planned 94 neonates could be
enrolled because of limited budget and time factors. Blinding could not
be done due to nature of the intervention. Both vitamin K1 and K3 were
utilized in the intervention group, as per availability in the drug
store of the institute. However, this is not likely to affect the
outcome as both vitamin K1 and K3 have equal efficacy in preventing
vitamin K deficiency [8]. Further, PIVKA-II level assessment, ELISA kits
from two different manufacturers were used due to financial constraints
and quantitative PIVKA-II levels could not be done in all the enrolled
neonates due to non-availability of blood serum samples.
High levels of PIVKA-II of fetal origins may persist
upto first 48 to 72 hours after birth in the peripheral blood so,
PIVKA-II levels were evaluated only on the 7 th
day of antibiotic therapy to avoid false positive results [8]. Such high
proportion of neonates with abnormal PIVKA-II levels has not been
reported in the published literature. In the prospective observational
study by Najmaldin, et al. [4], 49 infants (<6 weeks) admitted in
surgical ICU on antibiotics were enrolled. They observed detectable
PIVKA-II levels in 41% of the study population but the method of
PIVKA-II estimation and exact cut-off followed is not mentioned. De
Montalbert, et al. [7] enrolled 43 patients (3 months-23 years)
with cystic fibrosis on antibiotic therapy. They found abnormal PIVKA-II
concentrations in 33% of the patients but the method for PIVKA-II
estimation was not ELISA-based, and the cut-off used was also different.
In a subset of neonates, where quantitative estimation was possible, we
noticed PIVKA-II levels in the range of 900-1000 ng/ml. Such high levels
have not been previously described in the literature. In a previous
study from our center including term healthy neonates, median PIVKA-II
levels were in the range of 1.9-2 ng/mL at 72 hours of age [8]. Possible
reasons for this difference could be use of kits from a different
manufacturer, and that majority of patients in our study population were
born preterm. The overall prevalence of coagulopathy was only 6% in the
study population, with no difference between the two groups. In the
previous studies by Aziz. et al. [9] and Bhat, et al. [3]
in the older children, the prevalence of coagulopathy at 10 days of
antibiotic therapy was 15% and 33%, respectively. The overall low
prevalence of coagulopathy in our study could be due to the fact that
all our neonates received prophylactic vitamin K at birth and all
neonates with clinical bleeding before 7th day of antibiotic therapy
were excluded.
We conclude that neonates with sepsis receiving
antibiotics for 7 or more days have universal biochemical vitamin K
deficiency even after vitamin K administration, and question the utility
of this practice in neonates on prolonged antibiotic therapy. However,
it also raise questions about the usefulness of PIVKA-II measurement for
assessing vitamin K deficiency in neonates. There is a need to establish
normal values and standards of PIVKA-II measurements for assessing
subclinical vitamin K deficiency in the newborn infant.
Contributors: AS: study conception and
implementation, data management and writing the manuscript;
MJS,AT,RS,SC,RA: supervised implementation of the study and contributed
to writing of the manuscript. All authors approved the final version of
manuscript, and are accountable for all aspects related to the study.
Funding: None; Competing interest: None
stated.
|
What is Already Known?
• Coagulopathy due to vitamin K deficiency is
reported to be a frequent complication in adult patients in the
medical/surgical intensive care unit as a result of
prolonged antibiotic therapy.
What This Study Adds?
• Deficiency of vitamin K (as assessed by
PIVKA-II levels >2 ng/mL) persists even after intramuscular
administration of 1 mg vitamin K in neonates with sepsis on
prolonged antibiotic therapy.
|
References
1. Lipsky JJ. Mechanism of the inhibition of the
gamma-carboxylation of glutamic acid by N-methylthiotetrazole-containing
antibiotics. Proc Natl Acad Sci USA. 1984;81:2893-7.
2. Crowther MA, McDonald E, Johnston M, Cook D.
Vitamin K deficiency and D-dimer levels in the intensive care unit: a
prospective cohort study. Blood Coagul Fibrinolysis. 2002;13:49-52.
3. Bhat RV, Deshmukh CT. A study of Vitamin K status
in children on prolonged antibiotic therapy. Indian Pediatr.
2003;40:36-40.
4. Najmaldin A, Francis J, Postle A, Griffiths DM,
Burge DM, Karran S, et al. Vitamin K coagulation status in
surgical newborns and the risk of bleeding. J Pediatr Surg.
1993;28:138-43.
5. Clarke P, Mitchell SJ, Wynn R, Sundaram S, Speed
V, Gardener E, et al. Vitamin K prophylaxis for preterm infants:
A randomized, controlled trial of 3 regimens. Pediatrics.
2006;118:e1657-66.
6. Pal S, Curley A, Stanworth SJ. Interpretation of
clotting tests in the neonate. Arch Dis Child Fetal Neonatal Ed.
2015;100:F270-4.
7. De Montalembert M, Lenoir G, Saint-Raymond A, Rey
J, Lefrère JJ. Increased PIVKA-II concentrations in patients with cystic
fibrosis. J Clin Pathol. 1992;45:180-1.
8. Chawla D, Deorari AK, Saxena R, Paul VK, Agarwal
R, Biswas A, et al. Vitamin K1 versus vitamin K3 for prevention
of subclinical vitamin deficiency: a randomized controlled trial. Indian
Pediatr. 2007;44:817-22.
9. Aziz F, Patil P. Role of prophylactic vitamin K in preventing
antibiotic induced hypoprothrombinemia. Indian J Pediatr. 2015;82:363-7.
|
|
|
 |
|

