|
|
|
Indian Pediatr 2018;55: 519-520 |
 |
Transbronchial Lung Cryobiopsy for Diagnosis of Pediatric
Interstitial Lung Disease
|
|
JT Srikanta 1,
S Swarna1, DS
Shylendra2 and
Ravindra Mehta1
From 1Apollo Hospitals, Bengaluru; and
2Manvi Eyes & General Hospital, Deshpande Nagar, Hubballi;
Karnataka, India.
Correspondence to: Dr JT Srikanta, Institute of
Pulmonology, Apollo Hospitals, Bengaluru, India.
Email: [email protected]
Received: January 02, 2017;
Initial review: April 10, 2017;
Accepted: March 08, 2018.
|
Background: Tissue diagnosis of Childhood interstitial lung diseases
is of paramount importance to outline management. Case
characteristics: A 10-year-old boy with prolonged cough, and
computed tomography of thorax with features suggestive of primary
Langerhans’s cell histiocytosis. Intervention: Transbronchial
cryobiopsy of lung using flexible cryoprobe, revealed a final diagnosis
of Surfactant protein C/ABCA3 deficiency. Message: Transbronchial
cryobiopsy of the lung can provide adequate lung tissue for a
categorical diagnosis of interstitial lung diseases in children.
Keywords: Childhood interstitial lung disease, Langerhan’s
cell histiocytosis, Lung biopsy, Video-assisted thoracic surgery.
|
|
T
he term ‘diffuse pediatric lung disease’ has been
used interchangeably with Childhood interstitial lung disease (ChILD)
[1]. In the work-up of ChILD, tissue diagnosis with surgical lung
biopsies/video-assisted thoracic surgery (SLB/VATS) is the gold
standard, but is associated with significant risks [2]. Other option
include Transbronchial forceps lung biopsy (TBFB), but has limitations
of small specimen, and crush artifacts [3]. Newer diagnostic options for
ChILD include Transbronchial Cryobiopsy (TBCB), which is increasingly
being utilized for diagnosis of interstitial lung disease (ILD) in
adults [4-6]. We report use
of TBCB for a categorical diagnosis in ChILD.
Case Report
A 10-year-old boy presented with history of dry
cough, exertional dyspnea and intermittent wheezing, with inability to
gain weight and height for four years. There was no significant
antenatal or perinatal history, or recurrent infections requiring
prolonged intubation or ventilation. There was no history of aspiration,
swallowing dysfunction, or any exposure to pets, birds, farm dust,
metallic dust, fumes, or animal dander.
On examination, he was afebrile, and had heart rate
90 beats per minute, respiratory rate 24 breaths per minute, blood
pressure 100/66 mm Hg, and oxygen saturation 95% on room air. The height
(119 cm) and weight (15 kg) were below 3rd
centile. Clubbing was present on all digits. Chest
examination showed basal bilateral fine crackles with end-expiratory
wheeze.
The complete blood count, coagulation profile and
urine analysis was normal. Pulmonary function tests showed mixed airway
disease (FVC 23.8%, FEV1 19.8%, FEV1/FVC 81% predicted) with significant
post-bronchodilator reversibility (30.5%). Echocardiography was normal.
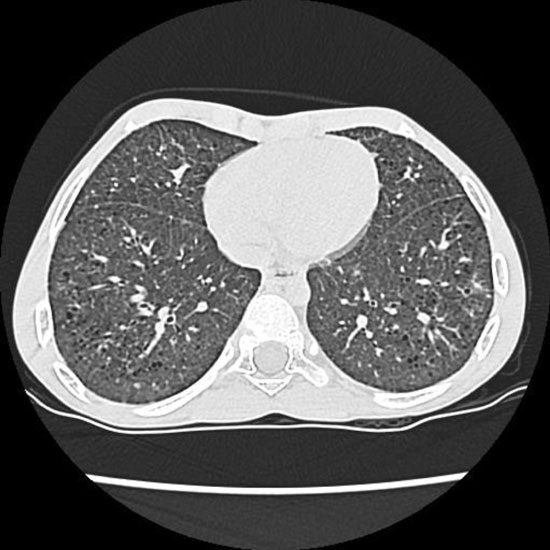
Chest computed tomography (CT) showed diffuse reticular opacities,
multiple bilateral small thin walled irregular cysts with relative basal
sparing, and diffuse ground glass opacities (Fig. 1).
A clinical diagnosis of primary Langerhan’s cell histocytosis (PLCH) was
considered.
 |
|
Fig. 1 Computed tomography of
chest shows diffuse reticular opacities with multiple bilateral
small thin walled irregular cysts.
|
For diagnosis, a comprehensive discussion of the
options (TBFB vs TBCB vs VATS) was done. The parents were
unwilling for VATS, and with the limitations of TBFB, a TBCB of the lung
was planned. The segment for biopsy was decided based on the CT of the
chest, targeting a significantly involved area (lingula). Rigid
bronchoscopy (ventilating bronchoscope, 6.5 mm) was performed under
general anesthesia. A flexible bronchoscope (diagnostic scope, channel
2.2 mm, Olympus Corporation) was introduced through the rigid scope to
facilitate passage of flexible accessories. A Fogarty balloon (5 mm) was
positioned at the entrance of the lingula, to restrict any bleeding to
that segment. A standard flexible cryoprobe (ERBE, Germany, 90 cm length
and 1.9 mm diameter) was introduced into the inferior lingula under
fluoroscopic guidance via the flexible bronchoscope. The biopsy
site was approximately 15-20 mm from the pleural surface. The biopsy
process involved cooling for 3-4 seconds, and adhering lung tissue to
the cryoprobe tip (cryoadhesion). The cryoprobe was then pulled with the
adherent specimen, removing the cryoprobe with the bronchoscope as a
unit. Simultaneously, with cryoprobe withdrawal, the appropriately
positioned Fogarty balloon was inflated to restrict any bleeding. The
frozen specimens (largest 27 mm 2)
were thawed in saline and sent for histopathology [7]. Recovery was
uneventful with no complications, and the child was discharged the next
day.
Microscopy of the sample (60 alveoli) showed focal
organizing pneumonia, indicated by tufts of fibroblasts extending into
the airspaces with interstital fibrosis and organizing lung injury. This
was suggestive of surfactant protein C deficiency or ABCA3
mutation, pending genetic diagnosis. Immunohistochemistry showed
negative CD1a stain, ruling out PLCH.
Discussion
SLB is considered the gold standard for the diagnosis
of ChILD’s [8], but is associated with significant morbidity (persistent
air-leak, persistent chest pain, cardiac arrhythmias, and infectious
complications) and mortality (2-4% at 90 days) [2]. TBFB pieces are too
small to define histology and TBCB offers an option between these two
modalities. The application of cryotherapy for lung biopsy is based on
the principle of cryoadhesion. Compressed carbon dioxide passing through
the probe expands suddenly at the tip, leading to rapid cooling (–
89ºC). This freezes the tissue in contact for biopsy, with preservation
of architecture due to cooling.
The most common complications reported in c ryobiopsy
for ILD are pneumothorax (4.5-7.5%) [5,9] and bleeding (1.4%) [6].
Pneumothorax risk can be minimized by fluoroscopy [6]. Bleeding, though
mild in most reports, can be controlled by Fogarty balloon tamponade
[6]. The rigid bronchoscope enables both cryoprobe and Fogarty to be
placed and utilized sequentially rapidly, which is important to control
bleeding.
The limitations of TBCB in the children include
difficulty in application. The rigid ventilating bronchoscope has to
accommodate both the flexible bronchoscope and the Fogarty balloon at
the same time, and hence a certain minimal size is essential. In our
experience, this requires a minimal rigid scope diameter of 6.5 mm.
Hence, it may not be possible to perform this procedure in children less
than 6 years of age [10].
We demonstrated TBCB to be possible and safe for
obtaining a categorical diagnosis in ChILD. TBCB provided adequate lung
tissue, and allowed rapid recovery and discharge.
Acknowledgments: Dr Megan K Dishop, Pediatric
Pathologist and Medical Director of Anatomic Pathology Children’s
Hospitals and Clinics of Minnesota, Minneapolis, USA for interpretation
of cryobiopsy sample.
Contributors: All authors were involved in
patient management, and contributed to the review of literature. All
authors approved the final version of the manuscript.
Funding: None; Competing interest: None
stated.
References
1. Fan LL, Deterding RR, Langston C. Pediatric
interstitial lung disease revisited. Pediatr Pulmonol. 2004;38:369-78.
2. Downey RJ. Complications after video assisted
thoracic surgery. Chest Surg Clin North Am. 1998;8:907-1097.
3. Kurland G, Noyes BE, Jaffe R. Bronchoalveolar
lavage and transbronchial biopsy in children following heart-lung and
lung transplantation. Chest. 1993;104:1043-8.
4. Babiak A, Hetzel J, Krishna G, Fritz P, Moeller P,
Balli T et al. Transbronchial cryobiopsy: A new tool for lung
biopsies. Respiration. 2009;78:203-8.
5. Kropski JA, Pritchett JM, Mason WR, Sivarajan L,
Gleaves LA. Bronchoscopic cryobiopsy for the diagnosis of diffuse
parenchymal lung disease. PLoS One. 8:e78674.
6. Poletti V, Casoni GL, Gurioli C, Ryu JH,
Tomassetti S. Lung cryobiopsies: A paradigm shift in diagnostic
bronchoscopy? Respirology. 2014;19:64554.
7. Langston C, Patterson K, Dishop MK. A protocol for
the handling of tissue obtained by operative lung biopsy:
Recommendations of the chILD pathology co-operative group. Pediatr Dev
Pathol. 2006;9:173-80.
8. Rothenberg S, Wagner J, Chang J, Fan L. The safety
and efficacy of thoracoscopic lung biopsy for diagnosis and treatment in
infants and children. J Pediatr Surg. 1996;31:100 4.
9. Fruchter O, Fridel L, Rosengarten D, Rahman NA,
Kramer MR. Transbronchial cryobiopsy in immunocompromised patients with
pulmonary infiltrates: A pilot study. Lung. 2013;191:619-24.
10. Masters IB, Ware RS, Zimmerman PV, Lovell B,
Wootton R, Francis PV, et al. Airway sizes and proportions in
children quantified by a video-bronchoscopic technique. BMC Pulmon Med.
2006;6:5.
|
|
|
 |
|

