|
|
|
Indian Pediatr 2016;53:
489-495 |
 |
Oral Antibiotics for Community–acquired
Pneumonia with Chest- indrawing in Children Aged Below Five
Years: A Systematic Review
|
|
Rakesh Lodha, Shivani Randev and Sushil K Kabra
From Department of Pediatrics, All India Institute of
Medical Sciences, New Delhi, India.
Correspondence to: Dr SK Kabra, Department of
Pediatrics, All India Institute of Medical Sciences,
New Delhi 110 029, India.
Email: [email protected]
|
Objectives: To determine the efficacy of oral antibiotics in
under-five children with pneumonia and chest indrawing.
Methods: We included controlled clinical trials
(randomized or quasi randomized) that compared the efficacy of oral
antibiotics versus parenteral antibiotics for treatment of
community- acquired pneumonia with chest-indrawing (severe pneumonia as
defined by the World Health Organization’s guidelines) in children below
60 months of age. Data were extracted and managed using RevMan software.
Main outcome variables were: treatment failure rate, relapse rate, death
rate, need for hospitalization, and severe adverse effects.
Results: We identified four randomized controlled
trials involving 4400 children who were diagnosed to have severe
pneumonia but were feeding well and not hypoxic. Baseline
characteristics of children in the two treatment arms (oral and
parenteral antibiotics) were similar. In two studies, oral antibiotics
were administered on an ambulatory basis, while in two, oral antibiotics
were used in hospitalized children. Failure rate in children receiving
oral antibiotics was 13% (288/2208) while that in children receiving
parenteral antibiotics was 13.8% (302/2183) (OR 0.93; 95% CI 0.78,
1.11). Failure rates were not affected by the type of oral antibiotic,
or presence of wheeze. Relapse rates, hospitalization or serious adverse
events were similar in the two groups.
Conclusion: Children with tachypnea with chest-indrawing
without signs/symptoms of very severe pneumonia may be treated with oral
antibiotics.
Key words: Ambulatory treatment, Amoxicillin, Management,
Outcome.
|
|
C
ommunity-acquired pneumonia is the leading cause
of under-five morbidity and mortality in developing countries. Out of
the 6.3 million deaths worldwide in children under five years of age in
the year 2013, pneumonia accounted for 14.9% of theses deaths.
To improve the case detection and to standardize the
management, WHO proposed simple classification for severity of
pneumonia. As per these guidelines, children with severe or very severe
pneumonia had to be treated with parenteral antibiotics [2]. As per the
guideline, all children with chest indrawing needed hospitalization for
parenteral antibiotics. This approach may be associated with multiple
problems [3]. Recent trials suggest that children with pneumonia and
chest indrawing may be treated with oral antibiotics [4-6]. We planned
systematic review of all the clinical trials evaluating oral antibiotics
in under-five children having community-acquired pneumonia with chest-indrawing.
Methods
All controlled clinical trials (randomized or quasi
randomized) that compared the efficacy of oral antibiotics with
parenteral antibiotics for treatment of community-acquired pneumonia
with chest-indrawing (severe pneumonia) for children below 60 months of
age were included. Severe pneumonia for the purpose of this review was
defined as cough or difficult breathing for less than two weeks; and
rapid breathing (defined as a respiratory rate of more than 50
breaths/min in children two months to 11 months old, and more than 40
breaths/min in children 12 to 59 months of age); and lower chest-indrawing
[2]. There were no language, regional or socio-economic restrictions.
Studies on children suffering from chronic pulmonary
diseases, immunodeficiency disorders, neurological disorders affecting
lung function, and cardiac disorders were excluded.
Types of interventions: Comparison of
antibiotics, in which at least one arm includes oral antibiotics in
hospital (inpatient or outpatient) or community-based setting. The other
arm may be parenteral therapy alone or switch therapy in which initial
parenteral treatment is followed by oral antibiotics to complete the
course. The antibiotics used for the oral and parenteral routes could be
different. Subgroup analyses were carried out for hospital-based
treatment/outpatient treatment and for type of oral antibiotics.
Outcome measures: Primary outcome measure
was ‘treatment failure’ defined as the presence of any of the following:
persistence of chest indrawing, at time of assessment within 2 weeks of
enrolment in the study, convulsions, drowsiness or inability to drink at
any time, respiratory rate above the age-specific cut-off point on
completion of treatment, or oxygen saturation of less than 90% (measured
by pulse oximetry) after completion of the treatment or mortality. Loss
to follow-up or withdrawal from the study at any time after recruitment
‘failure’ in the analysis was also considered as Secondary outcome
measures included: (a) ‘relapse’defined as
recurrence of symptoms/signs during follow up period following an
improvement in clinical signs and symptoms with treatment and declared
as cured; (b) death; (c) need for hospitalization; and, (d)
severe adverse effects.
Search strategy for identification of studies: We
searched the Cochrane Central Register of Controlled Trials: issue 4 of
2015 (The Cochrane Library), MEDLINE (1966 to April 2015) and EMBASE
(1980 to April 2015) by using appropriate terms (Web Table I).
We also searched bibliographies of the articles that
were selected for review to identify any additional trials not recovered
by the electronic searches.
Methods of the review: Abstracts of all articles
were read by two authors independently, and the relevant articles were
selected. Full text articles of selected studies were obtained. For
missing data, the corresponding author was contacted by e-mail. If there
was no response in two weeks time, we used the available information. A
scientist, not involved in the data extraction, concealed the
identifiers of the study by covering the titles, names of the authors on
the printed version of the articles, and assigned serial numbers to the
studies.
Data of baseline characteristics, and primary and
secondary outcome measures were extracted in a pretested performa by two
authors, independently. Differences in the data were resolved by
discussion with third author.
Statistical analysis: Analyses were carried out
using RevMan program (version 5.2). We assessed heterogeneity
using the RevMan software that gave I 2
values; we considered significant heterogeneity to be present if the I2
value was more than 30%. In case of heterogeneity between the studies,
efforts were made to explore the causes. Random effects model was used
for all analyses. Following subgroup analyses were also performed: (i)
Failure rates in children receiving oral amoxicillin in comparison to
parenteral penicillin/ ampicillin; (ii) Failure rates in children
receiving oral drug as cotrimoxazole in comparison to parenteral
penicillin; and (iii) Failure rates in ambulatory versus
hospitalized treatment regimen.
We planned to assess the publication bias by Funnel
plot in case sufficient number of trials were available. Quality of
included studies was assessed using the Cochrane Collaboration’s ’Risk
of bias’ tool [7] by two authors independently.
Results
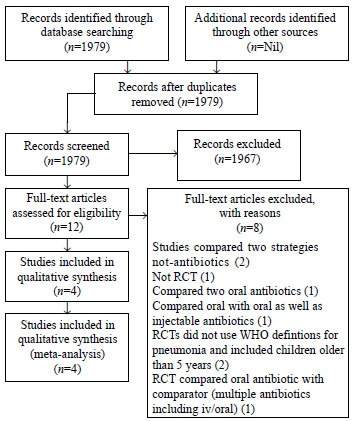
We identified a total of 1979 articles for the period
1966 to April 2015. After reviewing the abstracts of these articles,
full text articles of 12 studies were retrieved; of these, a total of 4
randomized controlled trials (RCTs) were identified for data extraction
(Fig. 1). Details of included studies are presented
in Table I.
 |
|
Fig.1 PRISMA Flow Diagram for
study selection.
|
TABLE I Details of Included Studies
|
Addo-Yobo, et al. [4] |
|
Methods |
This multicentre, randomized, open-label equivalency study
carried out at nine sites [Colombia, Ghana, India, Mexico,
Pakistan, South Africa (two sites), Vietnam, Zambia] with an aim
to determine whether oral amoxicillin and parenteral penicillin
were equivalent in the treatment of severe pneumonia in children
aged 3-59 months. |
|
Participants |
1702 children aged between 3 months to 59 months of either sex
with severe pneumonia based on case definition given by WHO. |
|
Interventions |
All patients were admitted for 48 hours. Patients received oral
amoxicillin 45 mg/kg/day in four divided doses for 5 days or
parenteral penicillin G 200000 IU /kg/day in four divided doses. |
|
Outcome |
Treatment failure was 19% in each group (161 patients,
penicillin; 167 amoxicillin; risk difference -0.4%; 95% CI -4.2
to 3.3) at 48 h. Infancy (age 3-11 months; odds ratio 2.72; 95%
CI 1.95 to 3.79), very fast breathing (1.94; 1.42 to 2.65), and
hypoxia (1.95; 1.34 to 2.82) at baseline predicted treatment
failure by multivariate analysis. |
|
Hazir, et al. [5] |
|
Methods |
Randomized, open-label equivalency trial was done at seven study
sites in Pakistan. |
|
Participants |
2100 children of either sex between 3 months to 59 months of age
with WHO defined severe pneumonia. |
|
Interventions |
Children either received parenteral ampicillin (100 mg/kg per
day in four doses) for 48 h, followed by 3 days of oral
amoxicillin (80-90 mg/kg per day; n=1012) in hospital or to
home-based treatment for 5 days with oral amoxicillin (80-90
mg/kg per day in two doses; n=1025). |
|
Outcome |
1048 were randomly assigned to hospitalization and injectable
ampicillin and 1052 to ambulatory treatment with oral
amoxicillin. As per intention to treat analysis cumulative
failure rates by day 6 in hospitalized and ambulatory treatment
was 105/1048 (10.0%) and 89/1052 (8.5%) respectively with a risk
difference of 1.6% (-0.9 to 4.0). Relapse rates by day 14 in
hospitalized and ambulatory treatment group were 31/943 (3.3%)
and 26/963 (2.7%) respectively with a risk difference of
0.6% (-0.9 to 2.1) |
|
Campbell et al. [6] |
|
Methods |
A quasi randomized controlled trial on children with cough with
chest indrawing (WHO defined severe pneumonia) in rural Gambia.
Children were assigned sequentially to one of the two treatment
groups (Oral co-trimoxazole or injection of procaine
penicillin). |
|
Participants |
134 children, aged 1 month to 4 years, who presented with acute
respiratory illness for less than 1 week with signs of
respiratory distress (intercostal indrawing or nasal flaring),. |
|
Interventions |
Children in group A received a 5-day course of oral co-trimoxazole
on ambulatory basis. Those in group B received a single
intramuscular injection of fortified procaine penicillin
(procaine penicillin 4 mega units plus benzylpenicillin 1 mega
unit per vial) and a 5-day course of oral ampicillin on
ambulatory basis. |
|
Outcome |
There were no significant differences between the two groups in
any of the symptoms, signs, or laboratory findings (e.g., length
of illness, mean respiratory or heart rate, mean temperature,
presence of auscultatory or radiological changes consistent with
pneumonia, and blood culture isolation rate). There were no
significant differences between the two groups in terms of final
outcome at 2 weeks follow-up when assessed either by the mothers
or the clinician. |
|
Agweyu, et al. [8] |
|
Methods |
An open-label, multicenter, randomized controlled noninferiority
trial was conducted at 6 Kenyan hospitals. Eligible children
aged 2-59 months were randomized to receive amoxicillin or
benzyl penicillin and followed up for the primary outcome of
treatment failure at 48 hours. |
|
Participants |
Children aged 2-59 months with severe pneumonia as defined in
the 2005 WHO guidelines were recruited from 6 public hospitals
across Kenya. |
|
Interventions |
Eligible children were randomized to oral amoxicillin at dose of
40-45 mg/kg twice daily or intravenous/intramuscular benzyl
penicillin at 50 000 IU/kg 4 times daily for a minimum of 48
hours. |
|
Outcome |
Treatment failure by day 5 postenrollment was 11.4% and 11.0% and
rising to 13.5% and 16.8% by day 14 in the amoxicillin vs benzyl
penicillin groups, respectively. Four patients died (overall
mortality 0.8%) during the study, 3 of whom were allocated to
the benzyl penicillin group. The presence of wheeze was
independently associated with less frequent treatment failure. |
|
Comments |
Open label randomized controlled trial and included children
with co- morbidity including malaria, diarrhea, wheeze, and a
single convulsion in the presence of fever. |
Of the four studies included, one was multi-country
[4] [Colombia, Ghana, India, Mexico, Pakistan, South Africa (two sites),
Vietnam, Zambia]; and one study each were carried out in Pakistan [5],
Gambia [6] and Kenya [8]. Three studies used amoxicillin as the oral
antibiotic and Penicillin/ampicillin as parenteral antibiotics [4,5,8]
while one used co-trimoxazole as the oral antibiotic [6]. Eight studies
were excluded [9-16]. Reasons for exclusion are given in
Web Table
II. As the number of included studies was only four, the publication
bias could not be assessed by funnel plot. Quality of studies is
described in Web Fig. 1.
Baseline characteristics of included subjects:
A total of four RCTs (4400 children less than 60 months of age) were
included for analysis. All 4 RCTs enrolled children below 5 years of
age; one of these included children from 1 month to 4 years of age [6].
Data on the number of children between 1-2 months were not available
separately. Information on children below 1 year of age was available in
3 studies [4-6]; a total of 2389 out of 3873 children were below one
year of age. The number of children below one year of age in oral and
parenteral antibiotics group was 1205 and 1184, respectively. The
proportion of infants was similar (OR 1.03; 95% CI 0.86, 1.22) in the
two groups. Number of boys in oral antibiotic group and parenteral
antibiotic group were 1314 and 1280, respectively (OR 1.04; 95% CI 0.92,
1.17).
Information on wheezing was available in three
studies [4,5,8]. These excluded children with current wheeze with
history of asthma and if their lower chest indrawing resolved with
salbutamol inhalation. Information on those who had current wheeze that
did not resolve with salbutamol inhalation was not available separately
according to groups in one study [4]; however, information on numbers
developing wheeze at 48 hours was available. Number of children with
wheeze in amoxicillin group and penicillin/ampicillin group were 931 and
935, respectively (OR 1.03; 95% CI 0.79, 1.33)
Only one study [4] provided data on children with
weight-for-age <–2Z. Number of children with malnutrition in those
getting oral or parenteral antibiotics were 124 and 133, respectively
(OR 0.91; 95% CI 0.69, 1.18).
Study by Addo-Yobo, et al. [4] excluded
children who received antibiotics in recent past. Others [5,8] included
children receiving antibiotics in recent past. Number of children in
oral and parenteral groups who gave history of receiving antibiotics or
their urine showed antimicrobial activities in urine were 268 and 152,
respectively (OR 1.21; 95% CI 0.97, 1.50).
Etiological agents were identified in one study [4].
Respiratory Syncytial Virus (RSV) was isolated from nasopharyngeal
aspirates of children getting oral or parenteral antimicrobials in
196/769 (25.5%) and 183/759 (24.1%), respectively (OR 1.05, 95% CI 0.83,
1.32). Nasopharyngeal cultures for bacterial pathogens were positive for
S. pneumoniae and H. influenzae in 201/743 and 146/743,
respectively in oral antibiotic group and same was 217/743 and 145/739,
respectively in parenteral antibiotic group.
Three studies compared oral amoxicillin with
ampicillin or penicillin [4,5,8]. One study compared oral cotrimoxazole
with injectable procaine penicillin [6].
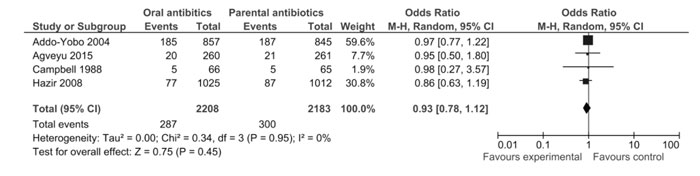
Treatment-failure rate: Failure rate in
children receiving oral antibiotics was 288/2208 (13%) while that in
children receiving parenteral antibiotics was 302/2183 (13.8%) (OR 0.93,
95% CI 0.78, 1.11) (Fig. 2).
 |
|
Fig. 2 Forest-plot for Primary outcome
of treatment failure rate.
|
Among three studies [4,5,8] involving 4166 children
(2145 in oral amoxicillin group and 2121 in parenteral antibiotics i.e.
penicillin/ampicillin, failure rates in children receiving oral
amoxicillin or parenteral antibiotics were 282/2142 (13.2%) and 295/2118
(13.9%), respectively (OR 0.93; 95% CI 0.78, 1.12).
Oral cotrimoxazole was used in one study [6]. The
failure rate was 6/66 (9.1%) and 7/65 (10.7%) in children receiving oral
cotrimoxazole or intramuscular procaine penicillin, respectively (OR
0.83; 95% CI 0.26, 2.61).
In two studies [4,8], all enrolled subjects were
admitted in beginning for atleast 48 hours. In one study [5],
hospitalized children received injectable ampicillin while those
receiving amoxicillin were treated on ambulatory basis. In one study
[6], both the groups were treated on an ambulatory basis. The odds ratio
of failure of treatment in the three studies was 0.97 [95%CI 0.77,
1.22], 0.86 [95% CI 0.63, 1.19], and 0.83 [95% CI 0.26, 2.61],
respectively.
Relapse rates: Only one study reported
relapse rates [5]. Number of patients who had relapse in oral
antibiotics and parenteral antibiotics groups was 25/948 (2.6%) and
31/925 (3.4%), respectively (OR 0.78; 95% CI 0.46, 1.33).
Hospitalization: One study [4] was
carried out in hospitalized children or at least they were admitted in
hospital for 2 days. In one study [5] children receiving parenteral
therapy were hospitalized at least for first two days; however, the
study does not report number of children in the oral antibiotic group
who required hospitalization; it suggests that those failed to treatment
were given alternative therapy. One study was carried out on ambulatory
basis [6]. In this study numbers requiring hospitalization in oral or
parenteral antibiotic group were 3/66 (4.5%) and 2/68 (2.9%),
respectively (OR 1.57; 95% CI 0.25, 9.72).
Death rates: Number of children who died in the
oral antibiotics and parenteral antibiotics groups were 5/2208 (0.2%)
and 15/1925 (0.8%), respectively (OR 0.3; 95% CI 0.11, 0.77).
Serious adverse events (SAE): SAE were
specifically reported in one study [4]. They noted SAE in 30 children (8
in amoxicillin group and 22 in penicillin group). The SAE were deaths in
12, rash in 5, diarrhea in 5, allergy to penicillin in 2, anemia and
malaria in one, severe malaria in 3 and unspecified events in 2.
Thirteen of these SAE were thought to be either possibly or probably
associated with the study drug, and treatment was discontinued or
changed in 12 of the 13 cases – all improved subsequently. None of the
deaths were attributed to study drug reaction.
Quality assessment: Three studies [4,5,8] were
assessed to be of good quality except that they were not blinded (Web
Fig. 1). These three studies compared oral amoxicillin with
parenteral antibiotics. The fourth study [6], comparing co-trimoxazole
with parenteral antibiotic, had inadequate information regarding the
sequence generation and allocation concealment; this was also an
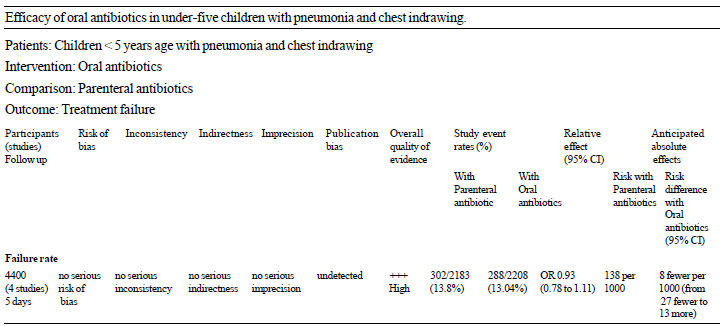
unblinded study. Using the GRADE framework, the available evidence is of
‘High quality’ (Table II).
|
TABLE II Grade Profile
|
 |
Discussion p>
The results from this systematic review suggest that
there is no significant difference in the outcome (failure rates and
relapse rates) of pneumonia with chest indrawing in under-five children,
between those treated with oral or parenteral antibiotics; the results
were not influenced by treatment in hospital or treatment in community,
the type of oral medications (amoxicillin or co-trimoxazole),
etiological agents (RSV positive or negative) and presence of wheeze.
Results suggest that children with pneumonia with chest indrawing (in
absence of danger signs and signs of very severe pneumonia) can be
treated with oral antibiotics. However, it is not possible to suggest a
single antibiotic that is most effective. In present review, it was not
possible to directly compare co-trimoxazole with amoxicillin.
All four included studies were carried out in
low-to-middle income group of countries. Three studies were carried out
in hospital (or partly in hospital) while one study was carried out
completely on ambulatory basis, exclusion of which did not change the
results. There were three more RCTs (that included children <60 months
of age along with older children) comparing oral and parenteral
treatment for severe pneumonia; these also suggest no difference in the
failure rates [11,12,16]. However, the WHO definitions were not used in
these studies. Two cluster-randomized controlled trials, carried out in
rural Pakistan, compared home treatment of severe pneumonia with
conventional treatment i.e. referral to hospital for parenteral
antibiotics [9,10]. Both the studies concluded that community case
management could result in a standardized treatment for children with
severe pneumonia, reduce delay in treatment initiation, and reduce the
costs for families and health-care systems. A multi-centric
observational study [14] also reported similar results. Only one
systematic review compared oral with parenteral antibiotic treatment
[17] and included only one study [6]. As part of comprehensive reviews
[18,19] on antibiotics for community acquired pneumonia in children,
subgroup analyses were carried out to document comparison of oral
versus parenteral antibiotics for treatment of severe pneumonia. In
these reviews, three studies [4-6] were included and reported that
failure rates were similar in the two groups. In the present review, we
included four studies that compared oral and parenteral antibiotics in
children below 60 months of age.
Mortality due to pneumonia may be affected by
underlying illness like acquired immune deficiency syndrome (AIDS),
congenital heart disease, severe malnutrition, and delayed intervention
due to health- seeking behavior. Results of present review may not be
applicable to countries with high rates of HIV infection. In one study
[4] included in present review, after interim analysis of results, a
modification in protocol was made to exclude children with suspected HIV
infection due to higher mortality rates in countries with higher HIV
infection rates. Therefore, the conclusions of present review may not be
applicable to countries with high HIV infection rates.
The availability of vaccination against S.
pneumoniae and H. influenzae (common organisms for community
acquired pneumonia in under-five children) is expected to change the
etiological agents as well as reduce mortality. This may change the
approach to management of pneumonia. However, in most middle- and
low-income group countries, the coverage of under-five children with
these vaccines is low [20]. Therefore, the present strategies for
management should be effective.
Present review has a limitation that the clinical
diagnosis of pneumonia was not confirmed by other investigations in the
included studies. However, it is a common practice to use only clinical
criteria for the diagnosis and management of pneumonia in high burden
settings.
Based on the results of the present review, we
conclude that children with pneumonia with chest indrawing (severe
pneumonia) from low- and middle- income countries and low rates of HIV
infection may be managed with oral antibiotics at home in absence of
danger signs or signs of very severe pneumonia, with monitoring by
health care workers.
Acknowledgement: Ms Sarah Thorning, Trials Search
Coordinator, Cochrane Acute Respiratory Infections Group, for assisting
us with literature search. Dr S Qazi for review and suggestions for
improving the quality of review.
Contributors: RL: data analysis, manuscript
writing, SR: literature search, data collection, manuscript writing,
SKK: literature search, data collection, analysis and manuscript
writing.
Funding: World Health Organization; Competing
interest: None stated.
|
What is Already Known?
• Children below 5 years of age with severe
pneumonia require hospitalization for treatment with intravenous
antibiotics
What This Study Adds?
• Children below 5 years of age with severe
pneumonia can be managed with oral antibiotics at home in
absence of danger signs or signs of very severe pneumonia, with
monitoring by health care workers.
|
References
1. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE,
et al. Global, regional and national causes of child mortality in
2000-13, with projections to inform post-2015 priorities: An updated
systematic analysis. Lancet. 2015;385:430-40.
2. Integrated Management of Childhood Illness: A
WHO/UNICEF initiative. Bull World Health Organization. 1997;75:(suppl.
1).
3. Athanassa Z, Makris G, Dimopoulos G, Falagas ME.
Early switch to oral treatment in patients with moderate to severe
community-acquired pneumonia: A meta-analysis. Drugs. 2008;68:2469-81.
4. Addo-Yobo E, Chisaka N, Hassan M, Hibberd P,
Lozano JM, Jeena P, et al. Oral amoxicillin versus injectable penicillin
for severe pneumonia in children aged 3 to 59 months: a randomised
multicentre equivalency study. Lancet. 2004;364:1141-8.
5. Hazir T, Fox LM, Nisar YB, Fox MP, Ashraf YP,
MacLeod WB, et al; New Outpatient Short-Course Home Oral Therapy for
Severe Pneumonia Study Group. Ambulatory short-course high-dose oral
amoxicillin for treatment of severe pneumonia in children: a randomised
equivalency trial. Lancet. 2008;371:49-56.
6. Campbell H, Byass P, Forgie IM, O’Neill KP,
Lloyd-Evans N, Greenwood BM. Trial of co-trimoxazole versus procaine
penicillin with ampicillin in treatment of community-acquired pneumonia
in young Gambian children. Lancet. 1988;2:1182-4.
7. RevMan 2012 Review Manager (RevMan) [Computer
program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The
Cochrane Collaboration, 2012.
8. Agweyu A, Gathara D, Oliwa J, Muinga M, Edwards T,
Allen E, et al. for the Severe Pneumonia Study Group. Oral amoxicillin
versus benzyl penicillin for severe pneumonia among Kenyan children: A
pragmatic randomized controlled non-inferiority trial. Clin Infect Dis.
2015;60;1216-24.
9. Soofi S, Ahmed S, Fox MP, MacLeod WB, Thea DM,
Qazi SA, et al. Effectiveness of community case management of severe
pneumonia with oral amoxicillin in children aged 2-59 months in Matiari
district, rural Pakistan: a cluster-randomised controlled trial. Lancet.
2012;379:729-37.
10. Bari A, Sadruddin S, Khan A, Khan IU, Khan A,
Lehri IA, et al. Community case management of severe pneumonia with oral
amoxicillin in children aged 2-59 months in Haripur district, Pakistan:
a cluster randomised trial. Lancet. 2011; 378:1796-803.
11. Atkinson M, Lakhanpaul M, Smyth A, Vyas H, Weston
V, Sithole J, et al. Comparison of oral amoxicillin and intravenous
benzyl penicillin for community acquired pneumonia in children (PIVOT
trial): A multicentre pragmatic randomised controlled equivalence trial.
Thorax. 2007; 62:1102-6.
12. Bradley JS, Arguedas A, Blumer JL, Sáez-Llorens
X, Melkote R, Noel GJ. Comparative study of levofloxacin in the
treatment of children with community-acquired pneumonia. Pediatr Infect
Dis J. 2007;26:868-78.
13. Chowdhury EK, El Arifeen S, Rahman M, Hoque DE,
Hossain MA, Begum K, et al. Care at first-level facilities for children
with severe pneumonia in Bangladesh: A cohort study. Lancet.
2008;372:822-30.
14. Addo-Yobo E, Anh DD, El-Sayed HF, Fox LM, Fox MP,
MacLeod W, et al. Multicenter Amoxicillin Severe pneumonia Study (MASS)
Group. Outpatient treatment of children with severe pneumonia with oral
amoxicillin in four countries: the MASS study. Trop Med Int Health.
2011;16:995-1006.
15. Straus WL, Qazi SA, Kundi Z, Nomani NK, Schwartz
B, Pakistan Co-trimoxazole Study Group. Antimicrobial resistance and
clinical effectiveness of co-trimoxazole versus amoxycillin for
pneumonia among children in Pakistan: Randomised controlled trial.
Lancet. 1998;352:270-4.
16. Sidal M, Ođuz F, Unüvar A, Sarbat G, Neyzi O.
Trial of co-trimoxazole versus procaine penicillin G and benzathine
penicillin + procaine penicillin G in the treatment of childhood
pneumonia. J Trop Pediatr. 1994;40:301-4.
17. Rojas MX, Granados C. Oral antibiotics versus
parenteral antibiotics for severe pneumonia in children. Cochrane
Database Syst Rev. 2006;2:CD004979.
18. Kabra SK, Lodha R, Pandey RM. Antibiotics for
community-acquired pneumonia in children. Cochrane Database Syst Rev.
2010;3:CD004874.
19. Lassi ZS, Das JK, Haider SW, Salam RA, Qazi SA,
Bhutta ZA. Systematic review on antibiotic therapy for pneumonia in
children between 2 and 59 months of age. Arch Dis Child. 2014;99:687-93.
20. Global Routine Vaccination Coverage, 2011. MMWR.
2012;61:883-5.
|
|
|
 |
|

