|
|
|
Indian Pediatr 2015;52: 505 -514 |
 |
IAP Position Paper on Burden of Mumps in
India and Vaccination Strategies
|
|
Vipin M Vashishtha, *Sangeeta Yadav,
#Aashima Dabas, CP Bansal,
Rohit C Agarwal, Vijay N Yewale, Naveen Thacker, SS Kamath and Pravin J
Mehta
From the Indian Academy of Pediatrics, Advisory
Committee on Vaccines and Immunization Practices (ACVIP); and
Departments of Pediatrics, *Maulana Azad Medical College and #University
College of Medical Sciences, New Delhi; India.
Correspondence to: Dr Vipin M Vashishtha,
Convener, IAP Advisory Committee on Vaccines and Immunization Practices,
Mangla Hospital and Research Center, Shakti Chowk, Bijnor, Uttar Pradesh
246 701, India.
Email: [email protected]
|
Justification: Mumps, despite being a widely prevalent disease in
the country, is considered as an insignificant public health problem
mainly because of poor documentation of clinical cases and lack of
published studies. In the absence of adequate published data on disease
burden, Government of India has recently decided to introduce
measles-rubella (MR) vaccine in its National Immunization Program and
neglected mumps component.
Process: Following an IAP ACVIP meeting on
December 6 and 7, 2014, a detailed review of burden of mumps in India
along with vaccination strategies to control the disease was prepared.
The draft was circulated amongst the members of the committee for review
and approval. Revised final draft was later approved by IAP executive
board in January 2015.
Objectives: To provide a review of community
burden of mumps in India; and to discuss the vaccination strategies to
impress upon policymakers to include mumps vaccination in National
immunization program.
Recommendations : A total of 14 studies and two
media reports on mumps outbreak were retrieved. The outbreaks were
reported from all the regions of the country. Mumps meningoencephalitis
was responsible for 2.3% to 14.6% of all investigated hospitalized acute
encephalitis syndrome or viral encephalitis cases in different studies.
Data from Infectious Disease Surveillance (ID Surv) portal of IAP and
Integrated Disease Surveillance Program (IDSP) of Government of India (GoI)
were also reviewed. While a total of 1052 cases were reported by the
IDSurv, IDSP had investigated 72 outbreaks with 1564 cases in 14 states
during different time periods. Genotypes G (subtype G2) and C were found
to be main genotypes of the mumps virus circulating in the country.
Three studies studied serological status of young children and
adolescents against mumps, and found susceptibility rates ranging from
32% to 80% in different age groups.
Conclusions: Mumps poses a significant disease
burden in India. This calls for inclusion of mumps vaccine in the
National immunization program.
Keywords: Epidemiology, Measles-mumps-Rubella vaccine,
National immunization program, Prevention.
|
|
T
he Government of India (GoI) has announced its
decision to include rubella vaccine in form of a bivalent
Measles-Rubella (MR) vaccine in its Universal Immunization Program (UIP)
[1]. The two-dose MR vaccine shall be provided at 9 months in place of
stand-alone measles vaccine, and at 16-24 months along with first
booster of Diphtheria-Tetanus-Pertussis (DTP) vaccine [2]. The Indian
Academy of Pediatrics (IAP) has argued very strongly for the inclusion
of Measles-Mumps-Rubella (MMR) vaccine instead of MR vaccine, because it
considers the burden of mumps is also significant, and the same
logistics can take care of three instead of two vaccine preventable
diseases (VPDs) [2]. The main reasons why GoI has not considered mumps
for inclusion in UIP are: the disease is not considered a serious public
health issue, lack of published data on the community burden of mumps,
and lastly the higher cost of the MMR vaccine in comparison to MR
vaccine [3]. The Academy believes that the burden of mumps is
significant and merits control strategies at national level. However,
lack of published studies hampers efforts to launch nation-wide
preventive strategies. Use of MR vaccine in place of MMR vaccine is
considered a ‘missed opportunity’ to target a significant VPD that also
has significant teratogenic effects on the developing fetus. The main
objective of this paper is to provide a review of community burden of
mumps in India along with discussion on vaccination strategies to
control the mumps disease.
Burden of Mumps Disease
Background
Mumps remains a prevalent viral disease with more
than 90% cases going unreported. The ‘classic’ mumps illness is
characterized by fever and swelling of the parotid gland(s) that affects
children and adolescents, and may lead to serious complications.
However, only half of infected subjects develop classical disease, and
about 30% of the infections are asymptomatic; a significant number of
infections are atypical (without parotitis) [4]. Acute
meningoencephalitis, the commonest serious complication seen in children
and adolescents, occurs in 1-10% of patients with mumps parotitis, but
only 40-50% of patients with mumps meningoencephalitis, confirmed by
serology or virus isolation, have parotitis [5].
The other complications of mumps include
pancreatitis, transverse myelitis, orchitis, oophoritis, deafness,
facial palsy, ascending polyradiculitis, cerebellar ataxia, and mastitis
[4,5]. The infection in pregnant women may result in spontaneous
abortions during first trimester and aqueductal stenosis manifesting as
congenital hydrocephalous in the newborn [2,4].
Global burden
The burden of mumps remains high (100-1000
cases/100000 population) in countries which do not offer routine mumps
vaccination, with epidemic peaks every 2-5 years [4,6]. Of late, there
has been resurgence of mumps even in countries using mumps vaccine in
their national immunization programs (NIPs) [7-9]. According to a recent
study by Global Infectious Disease and Epidemiology Online Network
(GIDEON) which covers 12,102 outbreaks of 215 infectious diseases
involving 44 million cases in 219 countries between 1980 and 2013, mumps
has emerged a notable ‘newcomer’ amongst human-specific infections in
the last decade [10]. According to WHO, Southeast Asia Region (SEAR)
reported 36,352 cases of mumps in 2013 [11], but there is no information
on the cases reported from India.
Burden of mumps in India
Mumps, despite being a widely prevalent disease all
over the country, is considered as an insignificant public health
problem in India, mainly because of poor documentation of clinical cases
and lack of published studies. There is no nationally representative
data on incidence of the disease. In fact, no attempt is being made so
far to collect and review even the available data through various
avenues. This review is an attempt to fill this void.
Search strategy: A thorough search using
appropriate terms was conducted through PubMed, Google Scholar, Google,
EMBASE, and other search engines. References cited in review articles
and case reports were also reviewed. Virus isolation and genotyping
studies on mumps virus from institutes like National Institute of
Virology, Pune and Postgraduate Institute of Medical and Educational
Research, Chandigarh were also studied. Studies citing virological
investigations of acute encephalitis syndrome (AES) and acute febrile
encephalopathy or viral meningoencephalitis were also included. VPD
surveillance portals like IDSurv and IDSP were searched to collect data
on sporadic cases and outbreaks of mumps. Google books on mumps and
newspaper articles publishing outbreaks of mumps disease were also
scanned.
Serological susceptibility: Three studies, two
from Northern [12,13] and one from Southern India [14] studied
serological status of young children and adolescents against mumps. In
first study, almost 60% of children were found to be susceptible to the
mumps virus [12]. In the other study by the same researchers, around 80%
and 70% mumps susceptibility rates in children aged 9-10 months and
15-18 months, respectively were noted [13]. A study among from 790
students from Manipal reported 32% susceptibility to mumps [14].
Outbreak investigations and virological studies of
AES cases: A total of 14 publications in various journals, and two
media reports on mumps outbreak were retrieved. Table I
presents a summary of investigations of the mumps outbreaks in different
regions along with some studies that had identified mumps virus as an
etiological agent of AES [15-30]. Few studies from some premiere
institutes of the country have attempted isolating circulating genotypes
of mumps virus in these outbreaks [21,22,24]. The outbreaks are reported
from almost all the regions of the country (Fig. 1) and
number of cases ranged from 7 to 301(Table I). Both
‘classic’ mumps cases with parotitis and mumps meningoencephalitis are
described. Among the studies conducted on hospitalized individuals with
AES and acute febrile encephalopathy, mumps contributed 2.3% to 14.6% of
all investigated AES or viral encephalitis cases [27-30] (Table
I).
TABLE I Summary of Published Studies Evaluating Outbreaks of Mumps and Acute Encephalitis Syndrome (AES) in India
|
Study [Ref.] |
Time period |
Place |
Clinical profile |
Vaccination status |
|
Studies reporting on Mumps outbreak |
|
Geeta, et al. [15] |
1999- 2003 |
Calicut, |
301 children admitted with mumps, |
Not mentioned |
|
|
Kerala |
58% in 5-9 year old. |
|
John TJ [16]
|
Jan-Mar
2002 |
Thiruvananthapuram,
Kerala |
179 cases; 98 were in age group 5-9 |
Not mentioned
|
|
Ghatage , et al.[17] |
Dec2005- |
Sangli, Maharashtra |
10 cases with mumps meningo- |
9/10 received single dose of |
|
2006 |
|
encephalitis, age group 3-13 yr |
MMR at 15-18 months age |
|
Vandana, et al. [18] |
2005 |
Manipal, Karnataka |
8 cases of atypical mumps, |
All unimmunized |
|
|
|
50% between 5-13 yr of age |
|
|
Arshad , et al. [19] |
2007-2011 |
Pulwama, South |
55 cases of parotitis in age group of |
All unimmunized |
|
|
|
Kashmir, J&K |
4-12 yrs |
|
|
Saha, et al.[20] |
2009 |
Kolkata, WB |
104 cases, attack rate 4.7%, the |
Not mentioned |
|
|
|
highest and lowest being in 6-10 |
|
|
|
|
years (11.7%) and above 15 years |
|
|
|
|
(0.9%), respectively |
|
|
*Malayan et al. |
2011- 2012 |
Chennai, TN |
56 patients, 39 from pediatric age |
30 out of 56 were vaccinated; |
|
[21] |
|
|
group (<18 yrs) |
status of 26 patients unknown |
|
*Vaidya, et al. [22] |
March 2012 |
Osmanabad, |
village: 91 mumps cases, |
All unimmunized |
|
|
Maharashtra |
Aspinga 74% in 5-14 yrs. Pimpla |
|
|
|
|
village: 51 cases, 84.3% in 5-14 yrs. |
|
|
Samuel , et al. [23] |
February 2012 |
Ludhiana, Punjab |
7 cases of mumps among 200 dental |
All immunized |
|
|
|
students of dentistry with average |
|
|
|
|
age was 22.57 years (22-24 years) |
|
|
*Mishra, et al. [24] |
August 2011 |
Fatehgarh Sahib, |
20 school children with mean age |
All unimmunized |
|
|
Punjab |
9.7 yrs mostly females (91%), |
|
|
**Amrita KR [25] |
January 2012 |
Ernakulam district, |
95 cases among school children |
Not mentioned |
|
|
Kerala |
|
|
|
*Ghai A [26] |
Aug-Sept |
Mohali, Punjab |
23 of the 49 children at Government |
Not mentioned |
|
2013 |
|
Elementary School |
|
|
Virological studies of AES cases |
|
Kumar, et al. [27] |
1985 -1988 |
Lucknow, UP |
5 (2.3%) cases of mumps encephalo- |
Not mentioned |
|
|
|
pathy out of 215 AES cases |
|
|
Karmarkar, et al. |
Feb- 2004- |
New Delhi |
6 (14.6%) cases of mumps meningo- |
Not mentioned |
|
[28] |
2005 |
|
encephalitis out of 41 cases of viral |
|
|
|
|
encephalitis |
|
|
Beig, et al. [29] |
2004- 2006 |
Aligarh, UP |
9 (10.5%) of meningo-encephalitis |
Not mentioned |
|
|
|
out of 87 cases of acute encephalitis |
|
|
Jain, et al. [30] |
January 2011 |
Lucknow, UP |
138 (8.7%) of meningo-encephalitis |
Not mentioned |
|
to December 2012 |
|
out of total 1578 cases of AES >1 yr old, 13 (9.4%) died and 7
left with neurological disability. |
|
|
*Genotype studies; **Media reports; AES: Acute encephalitis
syndrome. |
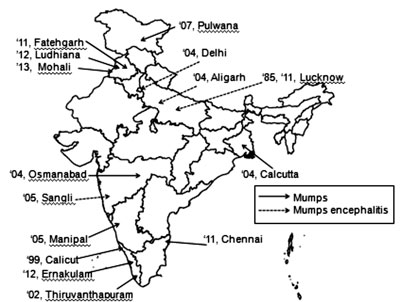 |
|
Fig. 1 Published studies evaluating
outbreaks of mumps and mumps menigoencephalitis amongst acute
encephalitis syndrome (AES) in India [15-30]. (Values represent
year and place).
|
| |
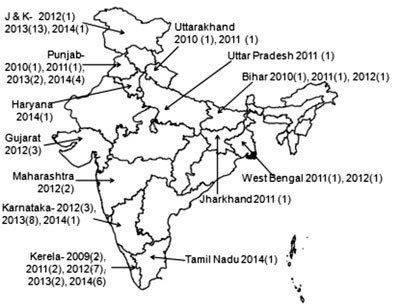 |
|
Fig. 2 Integrated Disease Surveillance
Program (IDSP) investigated outbreaks of mumps in India,
2009-2014 [33].
(Year, number of outbreaks in respective year).
|
The study of different circulating genotypes of mumps
virus in the community is a useful tool for identifying transmission
pathways and describing mumps epidemiology. There are 12 mumps genotypes
(A–N), and only one distinct serotype of mumps virus [31]. The studies
conducted on genotyping of circulating mumps virus found genotypes G
(subtype G2) and C prevalent in the studied outbreaks [21,22,24]. In one
report, two different genotypes, G and C were described to be
simultaneously circulating in two nearby villages of the same district
[22].
TABLE II IDSurv Data on Sporadic and Outbreak Cases of Mumps [32]
|
Time period |
Total number of cases |
Profile of cases |
Vaccination status |
Severity |
|
Jan 16, 2011 - |
808(7.6% of all the |
477 (59.1%) above 5 yr of age; |
84% unimmunized |
6% hospitalized |
|
Dec 16, 2013 |
reported VPDs) |
221 (27.4%) between 3-5 yr; |
|
(with complications) |
|
|
109 (13.5%) below 3 yr |
|
|
|
Nov 21, 2014 - |
244 (9.0 % of all the |
143 (58.6%) above 5 yr of age; |
61% unimmunized |
All outpatient cases |
|
Feb 20, 2015 |
reported VPDs) |
75 (30.7%) between 3-5 yr; |
|
without any mortality |
|
|
26 (10.7%) below 3 yr |
|
|
Infectious Disease Surveillance (IDSurv) portal of
IAP: Mumps is one of the ten infectious diseases included in the
web-based infectious disease surveillance system (IDSurv) launched by
IAP [32]. Passive reporting of the cases is done by IAP-member
pediatricians based in different cities and towns of the country.
Table II presents key features of reported cases during two
different periods. The data shows that majority of the reported mumps
cases are above 5 year of age, and are unimmunized. The reported mumps
cases reflect significant burden of the disease in the community; they
either exceed or equal the overall measles cases reported during these
time periods. The cases reported through this site represent both
sporadic and outbreak cases occurring throughout the year. However, the
reported cases represent only a ‘tip of the iceberg’ since out of 23,000
members, only less than 10% are reporting to this site. Further, the
number of the members regularly reporting is very less.
Integrated Disease Surveillance Program (IDSP):
This program, a surveillance system of the Government of India to detect
and respond to disease outbreaks, collects data on epidemic-prone
diseases, including mumps, on weekly basis from its reporting units such
as health sub-centers, primary health centers, community health centers,
hospitals and medical colleges [33]. It has reported and investigated 72
outbreaks of mumps during the period of September 2009 to November 2014
[33]. The outbreaks are reported throughout the year, and from all
regions of the country (Fig. 2). A total of 1564 cases
were reported in this period. Kerala, Jammu and Kashmir, Punjab, and
Karnataka had maximum number of cases (Fig. 3). Some of
these outbreaks were also investigated by other researchers and
published in journals [19,22,24].
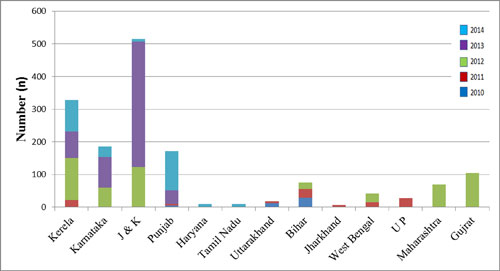 |
|
Fig. 3 Year- and state-wise
representation of number of cases investigated by Integrated
Disease Surveillance Program (IDSP) in different mumps outbreaks
in India, 2010-2014 [33].
|
Conclusions: The data presented highlight the
fact that mumps contributes significantly to morbidity in children in
India. The reported cases of mumps may actually be gross underestimate
of actual burden of mumps in the community, as majority are subclinical
infections which may go unnoticed and unreported. Also, most of the
symptomatic children may not seek health care, and go to faith healers
for advice [34], and hence are missed. The above review reflects the
burden of only ‘classic’ mumps and mumps meningoencephalitis, but there
is no data on other complications of the disease, including its
teratogenic effects.
Vaccination Strategies to Control Mumps
The above review indicates that mumps poses a
significant disease burden in India. Both sporadic cases and cyclic
outbreaks are regularly reported from all the regions of the country.
Safe and efficacious mumps vaccines are available in the country with an
indigenous large-scale producer. Near-elimination of mumps could be
achieved by adopting and maintaining good coverage of a two-dose
strategy in National immunization program [4]. Globally, the incidence
of mumps has reduced drastically in countries that have employed mumps
vaccination in their immunization schedules. Finland completely
eliminated natural transmission of mumps in 1996 [35]. At the end of
2007, 114 countries were administering mumps vaccine, compared with 104
countries at the end of 2002. However, as of 2012, 120 (62%) countries
have adopted routine mumps vaccination in their NIPs [36]. The reduction
in mumps incidence varies from 88% to 97% in countries adopting single
or two doses of vaccine, respectively [6]. A recent meta-analysis in
China found the overall vaccine effectiveness for mumps-containing
vaccine (either one dose or two doses) to be 85% (95% CI 76%-90%) from
cohort studies and 88% (95% CI 82%-92%) from case-control studies [37].
According to the WHO, vaccination strategies targeting mumps control
should be closely integrated with existing measles elimination and
rubella control [4]. A high coverage with the mumps vaccine is required
to offset any undesirable epidemiological shift of the disease to older
age groups with resultant higher rates of serious disease and
complications.
Efficacy and effectiveness of mumps vaccines: At
least 13 different strains of mumps virus, including Jeryl-Lynn,
Leningrad-3, Leningrad-Zagreb and Urabe Am9 are used for the development
of live attenuated mumps vaccines around the world. Though their
protective efficacy and effectiveness vary to some extent, but overall
they can protect about 80% of recipients [38]. Most of the strains
result in ³90%
seroconversion and/or short-term protective efficacy after
administration of single dose, but the long term effectiveness of one
dose is reported to be much lower (60-90%) [4]. The Jeryl–Lynn (Priorix
by GSK) and Leningrad–Zagreb (Tresivac by Serum Institute of India)
strains are used in the production of mumps vaccines available in India.
According to a recent Cochrane review analyzing data from 64 MMR vaccine
studies, the effectiveness of one dose of MMR in preventing clinical
mumps was found to be 69%-81% for Jeryl- Lynn-containing vaccines and
70%-75% for Urabe AM9-containing vaccines [39]. The effectiveness of
other mumps vaccine strains is difficult to determine, owing to more
limited use and fewer studies. However, few older studies evaluated
effectiveness of Leningrad-3 and Leningrad–Zagreb strains in Russia and
Yugoslavia, respectively, reported comparable rates of around 91-99% for
the former and 97-100% for the latter [38].
There is no effectiveness data available from India
since mumps is not part of NIP and only few states and Union Territories
are providing mumps vaccine in form of MMR vaccine [2]. Though the MMR
vaccine is offered by private sector, the coverage and field-efficacy
data are not available. Yadav, et al. [13] reported high mumps
seropositivity rates (96-100%) with use of single dose of MMR vaccine in
Delhi children. In another Indian study conducted amongst 1-10 year old
children in Pune, a single dose of MMR (with Leningrad-Zagreb mumps
virus strain) was able to maintain mumps-specific IgG (seropositivity
rate) in 95% after 6 years [40]. On the other hand, in a study from
Chennai, mumps component in the MMR vaccine was found to have low
seropositivity; only 15% of vaccinees with a single dose of MMR tested
positive for mumps-specific IgG [41].
Safety of mumps vaccines: Overall, all the
available mumps strains are considered safe; only mild adverse reactions
are noted. Few cases of mild, self-limiting aseptic meningitis have been
reported following the use of the Urabe Am9, Leningrad–Zagreb, Hoshino,
Torii and Miyahara strains [4]. The highest risk of association with
aseptic meningitis was observed within the 3rd week after immunization
with Urabe-strain (RR14.3; 95% CI 7.9, 25.7), and within the 3rd (RR
22.5; 95% CI 11.8, 42.9) or 5th (RR 15.6; 95% CI 10.3, 24.2) week after
immunization with the Leningrad-Zagreb strain [38]. Very low rates of
aseptic meningitis cases have been associated with the use of the
Jeryl–Lynn and RIT4385 strains [4]. However, due to the variability of
the methods used in the different studies, no clear conclusion can be
drawn on the differences in risk for aseptic meningitis among various
strains, and in 2006 the WHO Global Advisory Committee on Vaccine Safety
(GACVS) concluded that in terms of safety, all mumps vaccine
preparations are acceptable for use in immunization programmes [4].
Recent resurgence of mumps among vaccinated
individuals: A resurgence of mumps after a single vaccine dose has
been seen globally [42,43], following which a second dose of mumps
vaccine was introduced at 4-5 years [44,45], preferably in combination
with measles and rubella vaccines. The effectiveness of two doses is
estimated to be between 69% and 95% [39,46,47]. However, despite
reasonable vaccine effectiveness, outbreaks of mumps have been reported
globally, mainly in older children (Table III)
[48-56].
TABLE III Summary of Studies Evaluating Outbreaks of Mumps Globally, 2000-2012.
|
Researcher |
Place, Year
|
Clinical profile |
Vaccination status |
Sane, et al. [48]
|
Netherlands,
2009-2012
|
Annual incidence per 100,000-4.5 in 13-17-year age group,
21.4 in 18-25-year age group
|
All vaccinated. 67.7%
received two doses
|
Walker, et al. [49]
|
Scotland,
2010-2011 |
119 cases. Median age 20 yr
|
44.5% received single dose, 27.7%
received
two doses |
|
Nelson, et al. [50] |
Guam, US, 2010 |
505 cases. School age children
|
93% received two doses
|
|
Bangor-Jones, et al.
[51] |
Western Australia,
2007-2008 |
183 cases. 54% less than 20 yr |
67% received single dose, 52% two
doses |
|
Gonzalez, et al. [52] |
Spain, 2008 |
116 cases over 7 months. 68.9% school age |
Vaccine coverage >90% with two
dose effectiveness as 99% |
|
Dayan, et al. [53] |
United States, 2006 |
6584 mumps cases- 83%
|
63% vaccinated: 10% single and
53%
|
|
|
college students |
two doses
|
|
Cohen, et al. [54] |
England, 2004 |
312 cases. Age 2-12 yr |
Vaccine effectiveness 88% for
single dose
|
|
|
|
and 96% for two doses
|
|
Hindeyeh, et al. [55] |
Palestine,
2004-2005
|
3871 mumps cases (parotitis). 76.3% in 5-15 yr age group |
Vaccination coverage >85%.
|
|
Reaney, et al. [56] |
Ireland, 2000 |
332 cases positive, 95% in 9-19
yr age |
55% had received one dose, 1% two
doses |
Causes of resurgence and waning of immunity:
Primary vaccine failure is unlikely to be a cause of these outbreaks in
vaccinated individuals. Low coverage and use of single dose of mumps may
have been contributing factors in some outbreaks, but outbreaks are
reported even amongst vaccinees with two doses and with good coverage.
Hence, there is definite waning of protective immunity following either
single or two doses of mumps vaccination. However, waning after two
doses is not as dramatic as after single dose [38].
Waning of immunity following large-scale mumps
vaccination in few industrialized countries can be attributed to lack of
natural boosting due to highly successful vaccination programs. With
near elimination of mumps in several countries that have achieved high
levels of two-dose vaccine coverage, opportunities for boosting are
limited. Another reason could be poor B-cell memory responses induced by
mumps strain present in MMR vaccine. In a study, it was shown that
measles virus in MMR vaccine induced 3-fold higher levels of
virus-specific antibody-secreting cells than mumps virus [57]. Other
possible explanations could be high population density and contact rates
in colleges and universities, as well as antigenic differences between
the vaccine strain and the wild-type strain, possibly permitting immune
escape [38,41]. To counteract occurrence of outbreaks amongst highly
vaccinated individuals, a third dose of mumps vaccine is being
contemplated, though the current evidence for its use is still lacking
[58]. Further, adding third dose may not be cost-effective as far as
mumps control is concerned.
Timing and scheduling of mumps vaccine: IAP has
recently revised its recommendations on MMR vaccination with first dose
at 9 months in place of stand-alone measles vaccine, and second at 15
months of age [59]. The timing of the first dose was initially advocated
beyond 12 months due to possible interference by maternal antibodies.
However, as per Indian data, a significant part of the infant population
remains susceptible to mumps [13]. Wang, et al. [60] found 60-63%
seroprevalence rates in Chinese infants. The seropositivity increased to
92% at 2-4 year after vaccination, but declined again at 5-9 years [60].
Furthermore, the new recommendations also conform to the SAGE guidelines
[61], which include (i) for countries introducing or using
rubella vaccine, it must be given in combination with the first dose of
measles containing vaccine (MCV) (as MR or MMR); (ii) in
countries using rubella containing vaccine (RCV) and a two-dose schedule
of MCV, both doses should be of the same formulation [61]. There are
many studies, both from India and from other countries, demonstrating
efficacy and safety of MMR vaccine given at 9 month of age and
comparable seroconversion rates were seen at 9 months and 12-15 months
across different studies, implying minimum risk of interference of
maternal antibodies [13, 62-67]. Redd, et al. [68] reported that
response to mumps strain varied little by age of the child or birth year
of the child’s mother when immune responses to MMR vaccine given at 9,
12 or 15 months were compared [68]. Among 240 Indian children who
received MMR at 9-10 months or 15-18 months of age, seroconversion of
mumps was comparable in both groups (100% and 96%, respectively) [13].
Additionally, lowering the age of first dose would have better outreach
[2]. Therefore administering the first dose before 12 months may be a
prudent choice.
Cost-effectiveness analysis: Data from
industrialized countries have proved the cost-effectiveness of mumps
when translated to reduced school- and work-absenteeism and reduction in
associated long term complications and costs of associated
hospitalization. As per an economic analysis of mumps vaccination in US,
the average cost per case of mumps prevented was $3614, which was
greater than costs incurred with prevention of single measles case
($2207). The total annual costs averted by MMR vaccination was $
7,878,378,382 with a benefit-to- cost ratio of 0.49 [69]. Similarly, the
additional benefit of routine mumps vaccination exceeded additional
costs of vaccine in a cost-effectiveness analysis in Japan [70]. There
is no detailed cost-effectiveness analysis available for India.
Conclusions
IAP Committee on Immunization reiterates its firm
stand that mumps is a serious public health concern in India and the
disease should be targeted for control [2]. Control of mumps can be
linked to existing measles elimination and rubella control strategies.
The Committee believes that the move would not entail too much of extra
economic burden to the government considering the fact that mumps
vaccination can piggy-back on the existing measles and rubella
vaccination without employing extra logistics. Realizing the significant
community-burden of the disease in the community, the move should prove
to be a cost-effective exercise. It is high time that the government
realizes the current burden of mumps and need of mass vaccination for
its prevention and control. With the availability of a safe, effective,
indigenous and cost-effective vaccine, mumps should be immediately
included in the UIP as MMR vaccine in place of MR vaccine. Further,
there is an urgent need of initiating surveillance of clinical cases of
mumps all over the country and it should be declared as a ‘notifiable’
disease in India. The immunization coverage should be monitored, all
outbreaks should be investigated, and routine mumps surveillance should
be set up to evaluate the impact of vaccination.
Contributors: VMV reviewed the literature and
drafted the manuscript. SY and AD helped in literature search and
contributed to writing of manuscript. CPB, RCA, VNY, NT, SSK and PJM
reviewed the manuscript and provided intellectual inputs. All authors
approved the final version of manuscript.
Funding: None; Competing interests: None
stated.
ANNEXURE
Writing committee: Vipin M Vashishtha, Sangeeta
Yadav*, Aashima Dabas*, CP Bansal, Rohit C Agarwal, Vijay N Yewale,
Naveen Thacker, Sachindanand Kamath, Pravin J Mehta.
IAP Advisory Committee on Vaccines & Immunization
Practices, 2013-14: Office-bearers: CP Bansal (Chairperson),
Rohit Agarwal (Co-chairperson), Vijay Yewale (Co-chairperson), Vipin M
Vashishtha (Convener), Pravin J Mehta (IAP Coordinator), Members:
Shashi Vani, Anuradha Bose, Ajay Kalra, AK Patwari, Surjit Singh;
Consultants: Naveen Thacker, NK Arora, Rajesh Kumar, HPS
Sachdev, VG Ramchandran, Ajay Gambhir; Rapporteur: Panna
Choudhury.
Indian Academy of Pediatrics: Sachidananda
Kamath (President), Vijay N Yewale (Immediate Past-President), Sanjay K
Ghorpade (Vice-President), Pravin J Mehta (Secretary General), Bakul J
Parekh (Treasurer), Dheeraj Shah (Editor-in-Chief, Indian Pediatrics), P
Ramachandran (Editor-in-chief, Indian Journal of Practical Pediatrics),
AS Vasudev (Joint Secretary).
* Invited experts (outside of IAP ACVIP)
References
1. Government of India. The Three New Vaccines
Including indigenously Developed Rotavirus Vaccine to be Provided to all
Indian Children [Press release]. 2014 July 03. Available from:
http://pib.nic.in/newsite/PrintRelease. aspx?relid=106055. Accessed
December 26, 2014.
2. Vashishtha VM, Yewale V, Bansal CP, Mehta PJ. IAP
perspectives on measles and rubella elimination strategies. Indian
Pediatrc. 2014;51:719-21.
3. Government of India, Ministry of Health and Family
Welfare (Immunization Division). ICMR Expert Group Recommendations on
inclusion of Rubella vaccination, ICMR, May, 2012.
4. World Health Organization. Position paper. Mumps
virus vaccines. Wkly Epidemiol Rec. 2007;7:51-60.
5. Litman N, Baum SG. Mumps virus. In: Mandell
GL, Bennetts JE, Dolin R (eds). Principles and Practice of Infectious
Diseases; 6th ed, Philadelphia; Churchill Livingstone, 2003-2008.
6. Galazka A, Robertson S, Kraigher A. Mumps and
mumps vaccine: Global review.Bull WHO. 1999;77:3-14.
7. Atrasheuskaya AV, Kulak MV, Rubin S, Ignatyev GM.
Mumps vaccine investigation in Novosibirsk, Russia, 2002-2004. Clin
Microbiol Infect. 2007;13:670-6.
8. Centers for Disease Control and Prevention (CDC).
Update: Mumps Outbreak - New York and New Jersey, June 2009-January
2010. MMWR Morb Mortal Wkly Rep. 2010;59:125-9.
9. Kutty PK, McLean HQ, Lawler J, Schulte C, Hudson
JM, Blog D, et al. Risk factors for transmission of mumps in a
highly vaccinated population in Orange County, NY, 2009-2010. Pediatr
Infect Dis J. 2014;33:121-5.
10. Smith KF, Goldberg M, Rosenthal S, Carlson L,
Chen J, Chen C, et al. Global rise in human infectious disease
outbreaks. JR Soc Interface. 2014;11:20140950.
11. World Health Organization. Global and Regional
Immunization Profile; South-East Asia Region. Available from:http://www.who.int/immunization/monitoring
_surveillance/data/gs_seaprofile.pdf. Accessed January 3, 2015.
12. Chakravarti A, Yadav S, Berry N, Rastogi A,
Mathur MD. Evaluation of serological status of rubella and mumps in
children below five years. Indian J Med Res. 1999;110:1-3.
13. Yadav S, Thukral R, Chakravarti A. Comparative
evaluation of measles, mumps and rubella vaccine at 9 & 15 months of
age. Indian J Med Res. 2003;118:183-6.
14. Arunkumar G, Vandana KE, Sathiakumar N.
Prevalence of measles, mumps, rubella, varicella susceptibility among
helath science students in a University in India. Am J Ind Med.
2013;56:58-64.
15. Geeta MG, Kumar PK. Mumps-need for urgent action.
Indian Pediatr. 2004;41:1181-2.
16. John TJ. An outbreak of mumps in
Thiruvananthapuram district. Indian Pediatr. 2004;41:298-300.
17. Ghatage ST, Kakade GM. An outbreak of mumps
meningoencephalitis in Sangli district. Indian Pediatr. 2007;44:235.
18. Vandana KE, Arunkumar G, Bairy I. Role of
laboratory in rapid diagnosis of atypical mumps. Braz J Infect Dis.
2010;14:201-2.
19. Arshad AS, Shamila H, Khan I, Syed MA. Patterns
of mini-outbreaks of mumps at South Kashmir, Pulwama, India 2007-2011.
Nitte University Journal of Health Science. 2013;3:52-5. Available from:
http://nitte.edu.in/journal/March2013/POMOOM.pdf. Accessed
January 2, 2015.
20. Saha I, Haldar D, Paul B, Shrivastava P, Das DK,
Pal M, et al. An epidemiological investigation of mumps outbreak
in a slum of Kolkata. J Commun Dis. 2012;44:29-36.
21. Malayan J, Warrier A, Ramanan PV, Reddy SN,
Manickan E. Unnoticeable mumps infection in India: Does MMR vaccine
protect against circulating mumps virus genotype C? World Academy of
Science, Engineering & Technology 2012;6:1365-71. Available from:
http://waset.org/publications/1161/unnoticeable-mumps-infection-in-india-does-mmr-vaccine-protect-against-circulating-mumps-virus-genotype-c-
Accessed January 2, 2015.
22. Vaidya SR, Chowdhury DT, Kumbhar NS, Tomar R,
Kamble MB, Kazi MI. Circulation of two mumps virus genotypes in an
unimmunized population in India. J Med Virol. 2013;85:1426-32.
23. Samuel CJ, Thomas AM, Bhatia D. Mumps outbreak in
dental care providers in a North Indian dental college. CHRISMED J
Health Res. 2014 1:216-7. Available from: http://www.cjhr.org/text.asp?2014/1/3/216/138916
Accessed January 2, 2015.
24. Mishra B, Pujhari SK, Dhiman V, Mahalakshmi P,
Bharadwaj A, Pokhrel S, et al. Genotyping and subtyping of mumps
virus isolates from the Indian subcontinent. Arch Virol.
2013;158:2359-63.
25. Amritha KR. Sharp rise in mumps cases in
district. Available. from:
http://ibnlive.in.com/news/sharp-rise-in-mumps-cases-in-district/226432-60-122.html.
Accessed January 2, 2015.
26. Ghai A. Mumps outbreak at Mohali village. Tribune
News Service, Mohali, September 7, 2013. Available from:
http://www.tribuneindia.com/2013/20130908/cth1.htm. Accessed January
2, 2015.
27. Kumar R, Mathur A, Kumar A, Sethi GD, Sharma S,
Chaturvedi UC. Virological investigations of acute encephalopathy in
India. Arch Dis Child. 1990;65:1227-30.
28. Karmarkar SA, Aneja S, Khare S, Saini A, Seth A,
Chauhan BK. A study of acute febrile encephalopathy with special
reference to viral etiology. Indian J Pediatr. 2008;75:801-5.
29. Beig FK, Malik A, Rizvi M, Acharya D, Khare S.
Etiology and clinico-epidemiological profile of acute viral encephalitis
in children of western Uttar Pradesh, India. Int J Infect Dis.
2010;14:e141-6.
30. Jain P, Jain A, Kumar A, Prakash S, Khan DN,
Singh KP, et al. Epidemiology and etiology of acute encephalitis
syndrome in North India. Jpn J Infect Dis. 2014;67:197-203.
31. World Health Organization. Mumps virus
nomenclature update. Wkly Epidemiol Rec. 2012;87:217-24.
32. Infectious Disease Surveillance (IDSurv) by
Indian Academy of Pediatrics. Available from: http://idsurv.org/.
Accessed February 21, 2015.
33. Integrated Disease Surveillance Program (IDSP)
National Centre for Disease Control (NCDC), Directorate General of
Health Services, Ministry of Health and Family welfare, Government of
India. Available from: http://idsp.nic.in/idsp/IDSP/rcntobrk.pdf.
Accessed January 1, 2015.
34. Bhatnagar N, Kaur R, Gupta M, Sharma D.
Introducing combined measles, mumps and rubella vaccine in Chandigarh,
India: Issues and concerns. Indian Pediatr. 2014; 51:441-3.
35. Peltola H, Davidkin I, Paunio M, Valle M,
Leinikki P, Heinonen OP. Mumps and rubella eliminated from Finland.
JAMA. 2000;284:2643-7.
36. World Health Organization. Countries Using Mumps
Vaccine in National Immunization Schedule, 2012. Available from:
http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/mumps/en/
. Accessed January 6, 2015.
37. Wang H, Hu Y, Zhang G, Zheng J, Li L, An Z.
Meta-analysis of vaccine effectiveness of mumps-containing vaccine under
different immunization strategies in China. Vaccine. 2014;32:4806-12.
38. Rubin SA, Plotkin SA. Mumps vaccine. In
Plotkin SA, Orenstein WA, Offit PA (eds.) Vaccines. 6th edition.
Philadelphia: Saunders Elsevier. 2013. p. 419-46. .
39. Demicheli V, Rivetti A, Debalini MG, Di
Pietrantonj C. Vaccines for measles, mumps and rubella in children.
Cochrane Database Syst Rev. 2012;2:CD004407.
40. Raut SK, Kulkarni PS, Phadke MA, Jadhav SS, Kapre
SV, Dhere RM, et al. Persistence of antibodies induced by
measles-mumps-rubella vaccine in children in India. Clin Vaccine Immunol.
2007;14:1370-1.
41. Malaiyan J, Menon T. Low vaccine efficacy of
mumps component among MMR vaccine recipients in Chennai, India. Indian J
Med Res. 2014;139:773-5.
42. Hersh BS, Fine PEM, Kent WK, Cochi SL, Kahn LH,
Zell ER, et al. Mumps outbreak in a highly vaccinated population.
J Pediatr. 1991;119:187-93.
43. Briss PA, Fehrs LJ, Parker RA, Wright PF,
Sannella EC, Hutcheson RH, et al. Sustained transmission of mumps
in a highly vaccinated population: Assessment of primary vaccine failure
and waning vaccine-induced immunity. J Infect Dis. 1994;169:77-82.
44. van Loon FPL, Holmes SJ, Sirotkin BI, Williams
WW, Cochi SL, Hadler SC, et al. Mumps surveillance-United States,
1988–1993. MMWR CDC Surveill Summ. 1995;44:1-14.
45. Centers for Disease Control and Prevention.
Measles, Mumps, and Rubella—Vaccine Use and Strategies for Elimination
of Measles, Rubella, and Congenital Rubella Syndrome and Control of
Mumps: Recommendations of the Advisory Committee on Immunization
Practices (ACIP). MMWR. 1998;47RR-8.
46. Dayan G, Rubin S, Plotkin S. Mumps outbreaks in
vaccinated populations: Are available mumps vaccines effective enough to
prevent outbreaks. Clin Infect Dis. 2008;47:1458-67.
47. Domínguez A, Torner N, Castilla J, Batalla J,
Godoy P, Guevara M, et al. Mumps vaccine effectiveness in highly
immunized populations. Vaccine. 2010;28:3567-70.
48. Sane J, Gouma S, Koopmans M, de Melker H, Swaan
C, van Binnendijk R, et al. Epidemic of mumps among vaccinated
persons, the Netherlands, 2009-2012. Emerg Infect Dis. 2014;20:643-8.
49. Walker J, Huc S, Sinka K, Tissington A, Oates K.
Ongoing outbreak of mumps infection in Oban, Scotland, November 2012 to
January 2011. Euro Surveill. 2011;16:19803.
50. Nelson GE, Aguon A, Valencia E, Oliva R, Guerrero
ML, Reyes R, et al. Epidemiology of a mumps outbreak in a highly
vaccinated island population and use of a third dose of
measles-mumps-rubella vaccine for outbreak control–Guam 2009 to 2010.
Pediatr Infect Dis J. 2013;32:374-8.
51. Bangor-Jones RD, Dowse GK, Giele CM, van Buynder
PG, Hodge MM, Whitty MM. A prolonged mumps outbreak among highly
vaccinated Aboriginal people in the Kimberley region of Western
Australia. Med J Aust. 2009;191:398-401.
52. González PP, Barrios JA, Morales Serna JC. Study
of a population-wide epidemic outbreak of mumps virus G1 in Jerez de la
Frontera (Spain). Aten Primaria. 2012; 44:320-7.
53. Dayan G, Quinlisk P, Parker A, Barskey A, Harris
M, Schwartz J, et al. Recent resurgence of mumps in the United
States. NEJM. 2008;358:1580-9.
54. Cohen C, White J, Savage E, Glynn J, Choi Y,
Andrews N. Vaccine effectiveness estimates, 2004-2005 Mumps outbreak,
England. Emerg Infec Dis. 2007;13:12-7.
55. Hindiyeh MY, Aboudy Y, Wohoush M, Shulman LM, Ram
D, Levin T, et al. Characterisation of large mumps outbreak among
vaccinated Palestinian refugees. J Clin Microbiol. 2009;47:560-5.
56. Reaney EA, Tohani VK, Devine MJ, Smithson RD,
Smyth B. Mumps outbreak among young people in Northern Ireland. Commun
Dis Public Health. 2001;4:311-5.
57. Latner DR, McGrew M, Williams N, Lowe L, Werman
R, Warnock E, et al. Enzyme-linked immunospot assay detection of
mumps-specific antibody-secreting B cells as an alternative method of
laboratory diagnosis. Clin Vaccine Immunol. 2011;18:35-42.
58. Centers for Disease Control and Prevention.
Prevention of Measles, Rubella, Congenital Rubella Syndrome, and Mumps –
Recommendations by Advisory Committee on Immunization Practices (ACIP).
MMWR. 2013;62:1-40.
59. Vashishtha VM, Choudhury P, Kalra A, Bose A,
Thacker N, Yewale VN, et al. Indian Academy of Pediatrics (IAP)
recommended immunization schedule for children aged 0 through 18
years–India, 2014 and updates on immunization. Indian Pediatr.
2014;51:785-800.
60. Wang Z, Yan R, He H, Li Q, Chen G, Yang S, et
al. Difficulties in eliminating measles and controlling rubella and
mumps: A cross-sectional study of a first measles and rubella
vaccination and a second measles, mumps, and rubella vaccination. PLoS
One. 2014;9:e89361
61. Status Report on Progress towards Measles and
Rubella Elimination. SAGE Working Group on Measles and Rubella (17
October 2013). Available from:
http://www.who.int/immunization/sage/meetings/2013/november/Status_Report_Measles_Rubella21Oct2013_
FINAL.pdf . Accessed January 2, 2015.
62. Schoub BD, Johnson S, McAnerney JM, Wagstaff LA,
Matsie W, Reinach SG, et al. Measles, mumps, and rubella
immunization at nine months in a developing country. Pediatr Infect Dis
J. 1990;9:263-7.
63. Giammanco G1, Li Volti S, Salemi I, Giammanco
Bilancia G, Mauro L. Immune response to simultaneous administration of a
combined measles, mumps and rubella vaccine with booster doses of
diphtheria-tetanus and poliovirus vaccine. Eur J Epidemiol.
1993;9:199-202.
64. Singh R, John TJ, Cherian T, Raghupathy P. Immune
response to measles, mumps and rubella vaccine at 9, 12 and 15 months of
age. Indian J Med Res.1994; 100:155-9.
65. Forleo-Neto E, Carvalho ES, Fuentes IC, Precivale
MS, Forleo LH, Farhat CK. Seroconversion of a trivalent measles, mumps,
and rubella vaccine in children aged 9, 12 and 15 months. Vaccine.
1997;15:1898-901.
66. Klinge J1, Lugauer S, Korn K, Heininger U, Stehr
K. Comparison of immunogenicity and reactogenicity of a measles, mumps
and rubella (MMR) vaccine in German children vaccinated at 9-11, 12-14
or 15-17 months of age. Vaccine. 2000;18:3134-40.
67. Goh P, Lim FS, Han HH, Willems P. Safety and
immunogenicity of early vaccination with two doses of tetravalent
measles-mumps-rubella (MMR) vaccine in healthy children from 9 months of
age. Infection. 2007;35:326-33.
68. Redd SC, King GE, Heath JL, Forghani B, Bellini
WJ, Markowitz LE. Comparison of vaccination with measles-mumps-Rubella
vaccine at 9, 12, and 15 months of age. J Infect Dis. 2004;189:S116-22.
69. Zhou F, Reef S, Massoudi M, Papania MJ, Yusuf HR,
Bardenheier B, et al. An economic analysis of the current
universal 2-dose measles-mumps-rubella vaccination program in the United
States. J Infect Dis. 2004;189 (Suppl 1):S131-45.
70. Sugawara T, Ohkusa Y, Taya K, Oikawa K, Haneda
N, Kikuchi K, et al. Cost-effectiveness analysis of routine mumps
immunization in Japan. Kansenshogaku Zasshi. 2007;81:555-61.
|
|
|
 |
|

