|
Diarrhea, the third leading
killer of children in
India today, is responsible for 13% of all
deaths in children <5 years of age and kills an
estimated 300,000 children in India each year [1]. Rotavirus is the
leading cause of severe diarrhea in Indian children under 5, and has
been projected to cause 457,000 to 884,000 hospitalizations, 2,000,000
outpatient visits, and 122,000-153,000 deaths annually [2]. The
objectives of this paper are to review the epidemiology of rotavirus in
India, describe currently available and candidate rotavirus vaccines,
and examine the potential impact and cost-effectiveness of a national
rotavirus vaccination program.
EPIDEMIOLOGY
Burden of Rotavirus Diarrhea
Nationally representative data on the incidence of
severe rotavirus disease in India are lacking. However, a recent
prospective birth cohort study in Vellore rigorously characterized the
burden of rotavirus infection among children under 3 years of age [3].
The incidence of rotavirus diarrhea was 0.25 (95% CI 0.22, 0.29) per
child-year in children under 3 and 0.49 (0.42, 0.58) per child-year in
children under 1. 48% of children experienced at least one episode of
rotaviral diarrhea by age 3. In another cohort study in an urban slum
population in New Delhi [4], the overall annual incidence of rotavirus
hospitalizations in children <5 years of age was 337/100,000; incidence
for 1-year-olds was 1,270/100,000 with low incidence in the first 3
months of life; incidence for 2-year-olds was 534/100,000; and incidence
for 3-5 year olds was 12/100,000. This study highlights the importance
of young age in severe rotavirus infections, but because the study
looked at incidence of hospitalization and not disease, these numbers do
not represent the true incidence of rotavirus disease in India.
The contribution of rotavirus to diarrhea mortality
is typically inferred from diarrhea hospitalizations, which are
reasonably assumed to represent severe cases. The proportion of all
diarrhea hospitalizations caused by rotavirus has been evaluated in
numerous studies in India. On average, 34% (inter study variation (ISV):
19-50%) of all diarrhea hospitalizations are the result of rotavirus
infections [4-16] (Table I). The proportion of severe
diarrhea attributable to rotavirus has increased from an average of 25%
(ISV: 21-28%) in studies which were completed prior to 2000 to over 38%
(ISV: 19-50%) in studies that were completed after 2005. A similar
increase has been seen globally. It is postulated that improvements in
sanitation and use of antimicrobials have had a greater impact on
preventing bacterial and parasitic gastroenteritis (GE) than rotavirus
[17]. The reasons for this discrepancy are discussed later in the
article.
TABLE I Proportion of Diarrhea Cases Due to Rotavirus
|
Study Location |
Proportion
|
Total
|
Age |
Year |
|
RV+ |
Diarrhea
|
|
|
|
|
Cases |
|
|
|
Hospital Studies |
|
Pune[5] |
28.2% |
945 |
<5 |
1992-1996 |
|
Pune[6] |
28.3% |
628 |
<5 |
1993-1996 |
|
Chennai[7] |
22.5% |
745 |
<3 |
1995-1999 |
|
Vellore[8] |
21.0% |
602 |
<5 |
1995-1999 |
|
Kolkata[9] |
34.7% |
266 |
<4 |
1998-2000 |
|
Delhi[4] |
23.5% |
584 |
<5 |
2000-2001 |
|
Vellore[10] |
27.4% |
343 |
<5 |
2002-2003 |
|
Kolkata and Berhampur [11] |
36.3% |
545 |
<4 |
2003-2005 |
|
Lucknow[12] |
19.2% |
412 |
<3 |
2004-2008 |
|
Kolkata[13] |
37.3% |
668 |
<4 |
2005-2006 |
|
Delhi[14] |
36.9% |
862 |
<2 |
2005-2007 |
|
Nationwide[15] |
39.2% |
4243 |
<5 |
2005-2007 |
|
Manipur[16] |
49.9% |
489 |
<5 |
2005-2008 |
|
Summary |
33.6% |
11,332 |
|
|
|
Community Studies |
|
Pune[6] |
15.5% |
489 |
<5 |
1993-1996 |
|
Vellore[10] |
7.1% |
351 |
<2 |
2002-2003 |
|
Summary |
12.0% |
840 |
|
|
|
RV+: Rotavirus positive; Before 2000: 26.1 %; between
2000-2005: 29.1 %; after 2005: 38.3%. |
The proportion of diarrhea cases attributable to
rotavirus is notably lower in outpatient studies and community studies.
A previous review found that rotavirus accounts a median of 15% of
community diarrheal cases in India and 16% of diarrheal outpatients
[18]. Two additional studies, conducted in Pune and Vellore, found a
mean proportion of 12% (ISV: 7-16) [6, 10]. The higher prevalence of
rotavirus among hospitalized persons suggests that rotavirus
gastroenteritis is generally more severe than that of other etiologies,
an observation corroborated by the Vellore cohort, where the proportion
of diarrhea cases due to rotavirus increased with increasing disease
severity, from 11.5% in the least severe cases to 67.4% in the most [3].
Neonatal infections
The prevalence of rotavirus in neonates is high in
India, ranging from 22% to 73% [19-23]. Neonatal infections are commonly
asymptomatic, with 69-95% not showing overt signs of GE [21-24].
However, rotavirus infection has been detected significantly more in
neonates with diarrhea than in those without (55.5% vs 44.4%,
P<0.001) suggesting that neonates are not entirely immune to
rotavirus GE [22]. Viral shedding can begin as early as 2 days of age,
generally peaks around 3-6 days and resolves by 2 weeks of age; the
likelihood of acquiring an infection is related to length of stay in the
hospital after birth [19, 21,24]. Neonatal infections may be protective
against future rotavirus diarrhea, although results are conflicting.
This phenomena was first observed in a cohort of infants in Australia,
where neonatal infection was not protective against subsequent
reinfection but was protective against severe symptoms when reinfection
occurred [25]. In a cohort in New Delhi, infants with neonatal
infections suffered 46% fewer episodes of rotavirus diarrhea and 22%
fewer episodes of all-cause diarrhea in the first year of life [21].
Similarly, in a cohort of Bangalore children followed for 2 years, 2% of
neonatally infected children had rotavirus-associated diarrhea, while
39% of those not neonatally infected had symptomatic rotavirus
infections (P<0.001).[20] However, a larger study in Vellore did
not find any association between neonatal infection and either incidence
or severity of future rotavirus or all-cause GE [23]. The Bangalore and
Vellore studies limited their analyses to children infected with
particular RV types, strain I321 in Bangalore and G10P [11] in Vellore,
making it difficult to compare the two results. The role that neonatal
rotavirus infections play in disease epidemiology remains unclear,
although given the high burden of rotavirus disease observed in India
any protective effect seems likely to be minimal.
Age distribution
Most rotavirus disease in India occurs in the first
two years of life. In hospital-based studies, 87% (ISV: 58-95%) of all
rotavirus cases in children under 5 yr occurred by 18 months of age
[4-8, 10, 12, 15, 26]. Additionally, rotavirus GE is uncommon in the
youngest children; only 13% (ISV: 10-25%) of rotavirus cases in hospital
studies were in children younger than 6 months old. However, outpatient
and community studies found a higher proportion of cases (30%) in
children under 6 months [6, 10]. The difference in age distribution
between settings is likely largely a function of severity: in young
children, infection with rotavirus may be attenuated by the persistence
of maternal antibodies and thus, severe disease is less common.
Seasonality
While some studies in India have found no association
between rotavirus infection and time of year [10,16], most have observed
an increase in rotavirus-associated diarrhea during the winter months,
October to February, throughout the country [4,5,7,12,15,27,28]. The
observed proportion of rotavirus cases occurring in the cooler season
has ranged from 59% to 72%, with a median of 64%. The northern, more
temperate regions may exhibit stronger seasonality [15]. Nevertheless,
studies in Kolkata [29], Pune [5], and Chennai [7] have observed
seasonal effects despite their tropical climates, so the degree to which
seasonality varies by geography remains unresolved.
Serotype diversity
Rotavirus isolates from India are genetically
heterogeneous [4, 8-16, 30-35] (Web Table I). Such genetic
diversity is characteristic of Asia as a whole [36, 37], and
phylogenetic analyses of the VP7 (G) and VP4 (P) genes from India show
>95% homology with Asian reference strains for most isolates [16,27,35],
suggesting that rotavirus strains circulating in India are part of a
larger Asian transmission pool. The distribution of serotypes is similar
in northern and southern areas of the country and a few genotypes,
namely G1P[8] and G2P[4], often predominate in studies of non-neonates.
(Figs. 1, 2)
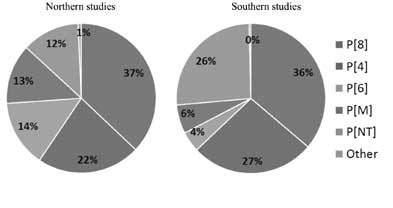 |
 |
|
Fig. 1 Rotavirus P serotype diversity
in Northern and Southern India.
|
Neonates are infected with a more limited spectrum of
viruses. In each study, one strain seems to predominate over other
strains, although the particular strain varies from study to study, with
G9P [11] [19, 21], G10P [11] [20, 38], and G12P[6] [27] having been
observed. Interestingly, several of these isolates have genetic homology
with non-human rotavirus, suggesting human and animal reassortant
viruses play a large role in neonatal infections [20,21,38].
Approximately 9% of all isolates are mixed
infections. The components of the mixed infections had a similar
distribution to the single-type infection distribution. Additionally,
roughly 13% of G types and 15% of P types were classified as untypable.
However, recent studies employing direct sequencing have illuminated the
importance of point mutations at the primer binding sites in causing
failures of PCR detection and genotyping [14, 33]. Sequencing the genome
of 16 previously untypable rotavirus isolates found that the strains
were a mixture of common genotypes, including G1, G8, G9 and G12 and
P[4] and P[8], with point mutations at the primer binding sites [33].
Treatment and Prevention
No specific treatment exists for rotavirus
gastroenteritis, and repeat infections are common in children [3].
Sanitation and hygiene improvements have had a tremendous impact on
diarrheal disease due to bacteria and parasites but less of an impact on
rotavirus disease. This is evinced by the persistence of rotavirus in
high income settings and the previously noted increase in the proportion
of GE cases due to rotavirus, and is thought to be due to transmission
through person-to-person contact, which persists even as fecal-oral
transmission diminishes [39]. Reduced osmolality oral rehydration
solution (ORS) effectively prevents and treats dehydration, and also
reduces diarrheal output [39], but the 2005 National Family Health
Survey found that nationally only 26% of children with diarrhea receive
ORS [40]. Unlike many other diarrheal pathogens, the proportion of
diarrhea caused by rotavirus does not vary widely between developed and
developing countries [41]. To date, the only specific prevention
strategy is immunization with rotavirus vaccines.
ROTAVIRUS VACCINES
Currently, two rotavirus vaccines are available on
the international market (Box) Rotarix
(GlaxoSmithKline, Rixensart, Belgium) is a monovalent
rotavirus vaccine (RV1) created by attenuating a highly antigenic strain
of human G1P[8] rotavirus [42]. RotaTeq
(Merck and Co., Whitehouse Station, USA) is a pentavalent
vaccine (RV5) created by reassorting G and P antigens from human
rotavirus, G1, G2, G3, G4 and P[1] with a bovine rotavirus strain [43].
While efficacy data from India are not yet available, both vaccines have
been tested extensively in a number of high and low income settings
which can be used predict the efficacy likely to be seen in India (Table
II) [39,44-46]. These vaccines are less effective against
medically attended rotavirus GE in lower income settings, varying in low
and lower middle income countries from 74% to 49%, with lower efficacy
seen in the lowest income countries [47]. Efficacies against severe
all-cause GE have ranged from 56% to 25%, with a less clear cut impact
between high and middle and low income countries (Table II)
[42, 47-50]. The efficacy of existing rotavirus vaccines in India are
likely to fall into the range for other low and middle income countries.
These vaccines appear to be cross protective against non-vaccine strains
[51], with similar efficacy against vaccine and non-vaccine strains
[47]. Vaccine efficacy, therefore, should be comparable between
countries with different serotype profiles, and be minimally affected in
serotype diverse countries such as India.
TABLE II Efficacy of RV1 or RV5 against Rotavirus Manifestations
|
Severe rotavirus gastroenteritis
|
|
Income level a |
Efficacy range |
Citations |
|
High
|
96-84% |
[39, 44] |
|
Upper middle
|
90-77% |
[39] |
|
Lower middle
|
74-55% |
[39, 45, 46] |
|
Lower
|
64-49% |
[39] |
|
Hospitalization due to all cause gastroenteritis |
|
Income status a |
Efficacy range |
Citations |
|
High |
56-30% |
[48-50] |
|
Middle or lower |
44-25% |
[42, 47] |
|
a World Bank 2008 classification; RV1:
monovalent and RV5: pentavalent rotavirus
vaccines. |
In high and middle income countries, recent
introductions of RV1 and RV5 have had remarkable impact on rotavirus
disease despite limited uptake and have provided both direct and
indirect protection in the under 5 population [51]. In the USA, a
42%-50% decrease in GE hospital admissions was seen in children aged 3
to 23 months during 2008 rotavirus season, two years after RV5
introduction, while only one-third of children <2 years had received one
or more doses of RV5 [52]. Additionally, a 28%-45% reduction in GE
hospitalizations was demonstrated during the rotavirus season in
children age-ineligible to receive RV5, suggesting significant indirect
protection [52]. In El Salvador, the introduction of RV1 was followed by
a 76% decrease in the number of hospitalizations due to rotavirus GE and
a 32% reduction in all-cause GE hospitalization within two years [46,
53]. Australia, which introduced both RV1 and RV5, saw a 74% decrease in
the same timeframe [54].
Two post-marketing studies have examined the impact
of vaccination against mortality from diarrhea. After RV1 introduction,
Mexico saw a 35% (95% CI: 29-39) reduction in the rate of diarrheal
deaths predominantly during the usual rotavirus season among children
age-appropriate for the vaccine [55]. After RV1 introduction in Brazil
in 2006, 30% (95% CI: 19-41) and 39% (95% CI: 29-49) decreases in
gastroenteritis mortality were noted in 2007 and 2008, respectively,
when compared to the mortality rates in 2004-2005 [56]. While suggestive
of substantial benefit, both studies were observational and the results
should be interpreted with caution.
Due to the association between intussusception and a
previously licensed live reassortant human-simian vaccine, both RV1 and
RV5 were under increased scrutiny for adverse events following
vaccination. During RV1 and RV5 development, >50,000 infants were
followed in clinical trials of each vaccine to determine if there is an
increased risk of intussusception, but none was found [57]. Emerging
data from post-marketing surveillance in Australia and Mexico suggest a
low level increased risk of intussusception (about 1-2 cases per 100,000
vaccinated) in the first week following vaccination [58]. While more
data are needed to fully understand intussusception risk across a range
of settings, at the level of risk observed, the benefits of vaccination
appear to greatly exceed the risks. At the rate observed in Mexico,
vaccination would result in an additional 20-40 cases of intussusception,
while preventing an estimated 700 deaths and 12,000 hospitalizations
from diarrhea [58]. Additionally, no increased rate of serious adverse
events such as fever, vomiting, or diarrhea was noted for either vaccine
[57]. Vaccine-associated disease has been noted in children with severe
combined immunodeficiency [59]. However, no increase in adverse effects
or mortality has been shown in HIV-positive children in South Africa
[57].
Based on the experiences of other developing
countries, a rotavirus vaccine introduced in India would be expected to
exhibit lower efficacy against rotavirus GE than seen in high income
countries, but still prevent a significant proportion of all-cause GE
mortality and hospitalizations. A small increased risk of
intussusception would be far outweighed by the number of diarrhea deaths
prevented.
Candidate Rotavirus Vaccines in India
Several candidate rotavirus vaccines are under
development in India. One candidate is based on a neonatal rotavirus
strain, 116E. This strain is a natural reassortant between a human
rotavirus virus G9P[11] strain with the VP4 protein from a bovine
rotavirus strain and was originally isolated from a neonate with an
asymptomatic rotavirus infection [60]. A recent randomized double
blinded placebo controlled trial for this strain has demonstrated that
the vaccine elicits a strong immune response in Indian children and was
not associated with an increase in adverse events [61]. Phase 3 trials
of the 116E vaccine in India are in progress and will provide data
regarding rotavirus vaccine efficacy in India. If successful, this
vaccine provides the possibility of a locally developed and tested
rotavirus vaccine for the Indian and international markets.
Other vaccine candidates under development are
various constructs of the UK bovine strain-based reassortant vaccine
developed by the US NIH [62]. The reassortant parent strains for the
vaccine have been licensed out to various manufacturers in India,
Brazil, and China. Indian-manufactured UK reassortant vaccines are
currently in phase 1 or 2 clinical trials.
Challenges to rotavirus vaccine performance in
developing countries such as India
Live oral vaccines have had an inconsistent
performance in India and other developing countries. For example, oral
polio vaccine (OPV) is less immunogenic and requires more doses to
protect children in India compared with children in the developed world
[63]. Similarly, the effectiveness of currently available rotavirus
vaccines (RV1 and RV5) is also inversely correlated (Table II)
to the childhood mortality levels in the countries where the clinical
trials were performed [64].
Reasons that live oral rotavirus vaccines are less
efficacious in developing countries are not fully understood. In
developing countries, higher levels of maternal rotavirus antibodies are
passively transferred to babies during gestation and persist in infancy
and some studies suggest that rotavirus vaccine neutralizing activity in
breast milk is higher in developing countries and may reduce vaccine
titer and adversely affect vaccine take[65], although breastfeeding has
not been shown to decrease the efficacy of RV5 [66]. Furthermore,
co-administration of OPV and rotavirus vaccines results in a small
decrease in the antibody response against rotavirus for both RV1 and
RV5, although the decrease is not significant in several studies after
the full course of both vaccines [39]. Neither of the currently
available rotavirus vaccines appears to interfere with polio immunity,
as anti-polio antibodies were similar regardless of if rotavirus vaccine
was given. Other reasons for poor vaccine performance could be a higher
prevalence of distinct medical conditions such as tuberculosis,
intestinal infections with other microorganisms, and malnutrition.
Potential impact and cost-effectiveness of rotavirus
vaccines in India
Estimates derived from available Indian child
mortality data suggest that, at current immunization levels, a national
rotavirus vaccination program in India would prevent 27,000 to 44,000
deaths in children <5 years of age annually [39, 67]. Rotavirus vaccine
would prevent 1 case of severe gastroenteritis disease for every 11
children immunized, and prevent one death for every 470 children
immunized [68]. The potential impact of rotavirus vaccines in India is
partially hindered by a relatively low proportion of children who
receive routine immunizations, which in 2006 was 52% for the third dose
of the diphtheria, tetanus, pertussis vaccine [40]. If full immunization
with rotavirus vaccine were reached, an additional 14,000 rotavirus
deaths each year could be prevented [39]. Improving the overall
performance of the immunization system is critical to the success of any
vaccine introduction.
The cost-effectiveness of a national rotavirus
vaccination program in India has been evaluated in two separate studies,
which reached similar conclusions [67, 68]. At a vaccine price of US
$1.00 per dose, the price set by Bharat Biotech [69], these models
estimated an incremental cost effectiveness ratio of $21.41 to $34 per
disability adjusted life year, which satisfies the WHO criterion for a
cost effective intervention. Even at the current UNICEF prices,
rotavirus vaccination is considered highly cost effective under WHO
criteria [67].
Summary
Rotavirus diarrhea causes substantial mortality and
morbidity in young children in India with the greatest burden among
children <2 years of age. Two rotavirus vaccines are currently available
on the international market. Additionally, at least two candidate
vaccines are under development by Indian manufacturers and may be
nationally licensed within 3-4 years. Despite the tremendous diversity
of rotavirus strains in India, rotavirus vaccines provide
cross-protection and have been shown to be effective against both
vaccine and non-vaccine strains. At current coverage levels of routine
childhood immunizations, the introduction of rotavirus vaccine in India
could prevent up to one third of rotavirus-related deaths [67].
Introduction of rotavirus vaccine into the national immunization program
of India at an affordable price would be a cost effective way to reduce
the tremendous morbidity and mortality due to rotavirus disease in
Indian children.
Note :The findings and conclusions in this report
are those of the authors and do not necessarily represent the views of
the Centers for Disease Control and Prevention (CDC).
Contributors: MS and UP conceived and designed
the review, and revised the manuscript. MS will act as guarantor of the
study. G Kahn, SF, and JT were responsible for initial review of
published studies and manuscript writing. G Kang, NG, GN, DS, RA, and
MC-S contributed substantially to manuscript revisions, including
providing expert opinion and additional studies for inclusion, and
interpreting results. The final manuscript was approved by all authors.
Funding: None;
Competing interests: None
stated.
Box: Rotavirus Vaccines Currently Available or Under Development
|
Type |
Company |
Schedule |
Status |
|
Live pentavalent human- |
Merck: Whitehouse Station,
|
Three doses |
International use
|
|
bovine reassortant (RV5) |
USA (Rotateq®) |
given with DTP
|
|
|
Live attenuated human
|
GlaxoSmithKline: Genval,
|
Two doses, given with |
International use
|
|
rotavirus (RV1) |
Belgium (Rotarix®) |
1st and 2nd DTP |
|
|
Live attenuated lamb |
Lanzhou Institute of Biological |
First dose given from 2
|
Licensed for use in
|
|
rotavirus (LLV) |
Products: Lanzhou, China
|
to 36 months, yearly booster
|
China
|
|
Serially passaged human
|
Bharat Biotech International
|
Under development |
Strains characterized.
|
|
neonatal rotavirus strain |
Limited: Hyderabad, India |
|
No phase 3 clinical trial
|
|
|
|
data available
|
|
Bovine human reassortant
|
Serum Institute of India and
|
Under development |
No clinical trial data
|
|
rotavirus vaccine |
Shantha Biotechnics |
|
available |
References
1. Bassani DG, Kumar R, Awasthi S, Morris SK, Paul
VK, Shet A, et al. Causes of neonatal and child mortality in
India: a nationally representative mortality survey. Lancet.
2010;376:1853-60.
2. Tate JE, Chitambar S, Esposito DH, Sarkar R,
Gladstone B, Ramani S, et al. Disease and economic burden of
rotavirus diarrhoea in India. Vaccine. 2009;27:F18-24.
3. Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J,
Sarkar R, Rehman AM, et al. Protective effect of natural
rotavirus infection in an Indian birth cohort. N Engl J Med.
2011;365:337-46.
4. Bahl R, Ray P, Subodh S, Shambharkar P, Saxena M,
Parashar U, et al. Incidence of severe rotavirus diarrhea in New
Delhi, India, and G and P types of the infecting rotavirus strains. J
Infect Dis. 2005;192:S114-9.
5. Kelkar SD, Purohit SG, Simha KV. Prevalence of
rotavirus diarrhoea among hospitalized children in Pune, India. Indian J
Med Res. 1999;109:131-5.
6. Kelkar SD, Purohit SG, Boralkar AN, Verma SP.
Prevalence of rotavirus diarrhea among outpatients and hospitalized
patients: a comparison. Southeast Asian J Trop Med Public Health.
2001;32:494-9.
7. Saravanan P, Ananthan S, Ananthasubramanian M.
Rotavirus infection among infants and young children in Chennai, South
India. Indian J Med Microbiol. 2004;22:212-21.
8. Kang G, Green J, Gallimore CI, Brown DW. Molecular
epidemiology of rotaviral infection in South Indian children with acute
diarrhea from 1995-1996 to 1998-1999. J Med Virol. 2002;67:101-5.
9. Das S, Sen A, Uma G, Varghese V, Chaudhuri S,
Bhattacharya SK, et al. Genomic diversity of group A rotavirus
strains infecting humans in eastern India. J Clin Microbiol.
2002;40:146-9.
10. Banerjee I, Ramani S, Primrose B, Moses P,
Iturriza-Gomara M, Gray JJ, et al. Comparative study of the
epidemiology of rotavirus in children from a community-based birth
cohort and a hospital in South India. J Clin Microbiol. 2006;44:2468-74.
11. Samajdar S, Varghese V, Barman P, Ghosh S, Mitra
U, Dutta P, et al. Changing pattern of human group A rotaviruses:
emergence of G12 as an important pathogen among children in eastern
India. J Clin Virol. 2006;36:183-8.
12. Mishra V, Awasthi S, Nag VL, Tandon R. Genomic
diversity of group A rotavirus strains in patients aged 1-36 months
admitted for acute watery diarrhoea in northern India: a hospital-based
study. Clin Microbiol Infect. 2010;16:45-50.
13. Samajdar S, Ghosh S, Chawla-Sarkar M, Mitra U,
Dutta P, Kobayashi N, et al. Increase in prevalence of human
group A rotavirus G9 strains as an important VP7 genotype among children
in eastern India. J Clin Virol. 2008;43:334-9.
14. Chakravarti A, Chauhan MS, Sharma A, Verma V.
Distribution of human rotavirus G and P genotypes in a hospital setting
from Northern India. Southeast Asian J Trop Med Public Health.
2010;41:1145-52.
15. Kang G, Arora R, Chitambar SD, Deshpande J, Gupte
MD, Kulkarni M, et al. Multicenter, hospital-based surveillance
of rotavirus disease and strains among indian children aged <5 years. J
Infect Dis. 2009;200:S147-53.
16. Mukherjee A, Chattopadhyay S, Bagchi P, Dutta D,
Singh NB, Arora R, et al. Surveillance and molecular
characterization of rotavirus strains circulating in Manipur,
north-eastern India: increasing prevalence of emerging G12 strains.
Infect Genet Evol. 2010;10:311-20.
17. Parashar UD, Gibson CJ, Bresse JS, Glass RI.
Rotavirus and severe childhood diarrhea. Emerg Infect Dis.
2006;12:304-6.
18. Ramani S, Kang G. Burden of disease &
molecular epidemiology of group A rotavirus infections in India.
Indian J Med Res. 2007;125:619-32.
19. Cicirello HG, Das BK, Gupta A, Bhan MK,
Gentsch JR, Kumar R, et al. High prevalence of rotavirus
infection among neonates born at hospitals in Delhi, India:
predisposition of newborns for infection with unusual rotavirus.
Pediatr Infect Dis J. 1994;13:720-4.
20. Vethanayagam RR, Ananda Babu M, Nagalaxmi KS,
Maiya PP, Venkatesh HA, Purohit S, et al. Possible role of
neonatal infection with the asymptomatic reassortant rotavirus (RV)
strain I321 in the decrease in hospital admissions for RV diarrhea,
Bangalore, India, 1988-1999. J Infect Dis. 2004;189:2282-9.
21. Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch
JR, Glass RI. Protection conferred by neonatal rotavirus infection
against subsequent rotavirus diarrhea. J Infect Dis. 1993;168:282-7.
22. Ramani S, Sowmyanarayanan TV, Gladstone BP,
Bhowmick K, Asirvatham JR, Jana AK, et al. Rotavirus
infection in the neonatal nurseries of a tertiary care hospital in
India. Pediatr Infect Dis J. 2008;27:719-23.
23. Banerjee I, Gladstone BP, Le Fevre AM, Ramani
S, Iturriza-Gomara M, Gray JJ, et al. Neonatal infection with
G10P[11] rotavirus did not confer protection against subsequent
rotavirus infection in a community cohort in Vellore, South India. J
Infect Dis. 2007;195:625-32.
24. Broor S, Ghosh D, Mathur P. Molecular
epidemiology of rotaviruses in India. Indian J Med Res.
2003;118:59-67.
25. Bishop RF, Barnes GL, Cipriani E, Lund JS.
Clinical immunity after neonatal rotavirus infection. A prospective
longitudinal study in young children. N Engl J Med. 1983;309:72-6.
26. Chitambar SD, Tatte VS, Dhongde R, Kalrao V.
High frequency of rotavirus viremia in children with acute
gastroenteritis: discordance of strains detected in stool and sera.
J Med Virol. 2008;80:2169-76.
27. Ray P, Sharma S, Agarwal RK, Longmei K,
Gentsch JR, Paul VK, et al. First detection of G12
rotaviruses in newborns with neonatal rotavirus infection at all
India Institute of Medical Sciences, New Delhi, India. J Clin
Microbiol. 2007;45:3824-7.
28. Phukan AC, Patgiri DK, Mahanta J. Rotavirus
associated acute diarrhoea in hospitalized children in Dibrugarh,
north-east India. Indian J Pathol Microbiol. 2003;46: 274-8.
29. Nair GB, Ramamurthy T, Bhattacharya MK,
Krishnan T, Ganguly S, Saha DR, et al. Emerging trends in the
etiology of enteric pathogens as evidenced from an active
surveillance of hospitalized diarrhoeal patients in Kolkata, India.
Gut Pathog. 2010;2:4.
30. Das S, Varghese V, Chaudhuri S, Barman P,
Kojima K, Dutta P, et al. Genetic variability of human
rotavirus strains isolated from Eastern and Northern India. J Med
Virol. 2004;72:156-61.
31. Jain V, Das BK, Bhan MK, Glass RI, Gentsch
JR. Great diversity of group A rotavirus strains and high prevalence
of mixed rotavirus infections in India. J Clin Microbiol.
2001;39:3524-9.
32. Sharma S, Ray P, Gentsch JR, Glass RI, Kalra
V, Bhan MK. Emergence of G12 rotavirus strains in Delhi, India, in
2000 to 2007. J Clin Microbiol. 2008;46:1343-8.
33. Sharma S, Paul VK, Bhan MK, Ray P. Genomic
characterization of nontypeable rotaviruses and detection of a rare
G8 strain in Delhi, India. J Clin Microbiol. 2009;47:3998-4005.
34. Tatte VS, Gentsch JR, Chitambar SD.
Characterization of group A rotavirus infections in adolescents and
adults from Pune, India: 1993-1996 and 2004-2007. J Med Virol.
2010;82:519-27.
35. Zade JK, Chhabra P, Chitambar SD.
Characterization of VP7 and VP4 genes of rotavirus strains:
1990-1994 and 2000-2002. Epidemiol Infect. 2009;137:936-42.
36. Santos N, Hoshino Y. Global distribution of
rotavirus serotypes/genotypes and its implication for the
development and implementation of an effective rotavirus vaccine.
Rev Med Virol. 2005;15:29-56.
37. Nelson EA, Bresee JS, Parashar UD, Widdowson
MA, Glass RI. Rotavirus epidemiology: the Asian Rotavirus
Surveillance Network. Vaccine. 2008;26:3192-6.
38. Iturriza Gomara M, Kang G, Mammen A, Jana AK,
Abraham M, Desselberger U, et al. Characterization of
G10P[11] rotaviruses causing acute gastroenteritis in neonates and
infants in Vellore, India. J Clin Microbiol. 2004;42:2541-7.
39. Chandran A, Fitzwater S, Zhen A, Santosham M.
Prevention of rotavirus gastroenteritis in infants and children:
rotavirus vaccine safety, efficacy, and potential impact of
vaccines. Biologics. 2010;4:213-29.
40. National Family Health Survey (NFHS-3),
2005–06: India: Volume I. Mumbai: IIPS; 2007.
41. Parashar UD, Burton A, Lanata C, Boschi-Pinto
C, Shibuya K, Steele D, et al. Global mortality associated
with rotavirus disease among children in 2004. J Infect Dis.
2009;200:S9-S15.
42. Ruiz-Palacios GM, Perez-Schael I, Velazquez
FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy
of an attenuated vaccine against severe rotavirus gastroenteritis. N
Engl J Med. 2006;354:11-22.
43. Vesikari T, Matson DO, Dennehy P, Van Damme
P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a
pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl
J Med. 2006;354:23-33.
44. Field EJ, Vally H, Grimwood K, Lambert SB.
Pentavalent rotavirus vaccine and prevention of gastroenteritis
hospitalizations in Australia. Pediatrics. 2010;126: e506-12.
45. Yen C, Armero Guardado JA, Alberto P,
Rodriguez Araujo DS, Mena C, Cuellar E, et al. Decline in
Rotavirus Hospitalizations and Health Care Visits for Childhood
Diarrhea Following Rotavirus Vaccination in El Salvador. Pediatr
Infect Dis J. 2010 Nov 2.
46. de Palma O, Cruz L, Ramos H, de Baires A,
Villatoro N, Pastor D, et al. Effectiveness of rotavirus
vaccination against childhood diarrhoea in El Salvador: case-control
study. BMJ. 2010;340:c2825.
47. Madhi SA, Cunliffe NA, Steele D, Witte D,
Kirsten M, Louw C, et al. Effect of human rotavirus vaccine
on severe diarrhea in African infants. N Engl J Med. 2010;362:
289-98.
48. Vesikari T, Karvonen A, Prymula R, Schuster
V, Tejedor JC, Cohen R, et al. Efficacy of human rotavirus
vaccine against rotavirus gastroenteritis during the first 2 years
of life in European infants: randomised, double-blind controlled
study. Lancet. 2007;370:1757-63.
49. Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM,
Quak SH, et al. Safety and efficacy of human rotavirus
vaccine during the first 2 years of life in Asian infants:
randomised, double-blind, controlled study. Vaccine.
2009;27:5936-41.
50. Muhsen K, Chodick G, Goren S, Shalev V, Cohen
D. The uptake of rotavirus vaccine and its effectiveness in
preventing acute gastroenteritis in the community. Vaccine.
2010;29:91-4.
51. Patel MM, Steele D, Gentsch JR, Wecker J,
Glass RI, Parashar UD. Real-world impact of rotavirus vaccination.
Pediatr Infect Dis J. 2011;30:S1-5.
52. Curns AT, Steiner CA, Barrett M, Hunter K,
Wilson E, Parashar UD. Reduction in acute gastroenteritis
hospitalizations among US children after introduction of rotavirus
vaccine: analysis of hospital discharge data from 18 US states. J
Infect Dis. 2010;201:1617-24.
53. Yen C, Armero Guardado JA, Alberto P,
Rodriguez Araujo DS, Mena C, Cuellar E, et al. Decline in
rotavirus hospitalizations and health care visits for childhood
diarrhea following rotavirus vaccination in EI salvador. Pediatr
Infect Dis J. 2011;30:S6-S10.
54. Paulke-Korinek M, Rendi-Wagner P, Kundi M,
Kronik R, Kollaritsch H. Universal mass vaccination against
rotavirus gastroenteritis: impact on hospitalization rates in
austrian children. Pediatr Infect Dis J. 2010;29:319-23.
55. Richardson V, Hernandez-Pichardo J,
Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano
CM, et al. Effect of rotavirus vaccination on death from
childhood diarrhea in Mexico. N Engl J Med. 2010;362:299-305.
56. Lanzieri TM, Linhares AC, Costa I, Kolhe DA,
Cunha MH, Ortega-Barria E, et al. Impact of rotavirus
vaccination on childhood deaths from diarrhea in Brazil. Int J
Infect Dis. 2011;15:e206-10.
57. Soares-Weiser K, Maclehose H, Ben-Aharon I,
Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus
diarrhoea: vaccines in use. Cochrane Database Syst Rev.
2010:CD008521.
58. WHO. Rotavirus vaccine and intussusception.
Weekly epidemiological record. 2011;86:38-40.
59. Patel NC, Hertel PM, Estes MK, de la Morena
M, Petru AM, Noroski LM, et al. Vaccine-acquired rotavirus in
infants with severe combined immunodeficiency. N Engl J Med.
2010;362:314-9.
60. Bhandari N, Sharma P, Glass RI, Ray P,
Greenberg H, Taneja S, et al. Safety and immunogenicity of
two live attenuated human rotavirus vaccine candidates, 116E and
I321, in infants: results of a randomised controlled trial. Vaccine.
2006;24:5817-23.
61. Bhandari N, Sharma P, Taneja S, Kumar T,
Rongsen-Chandola T, Appaiahgari MB, et al. A dose-escalation
safety and immunogenicity study of live attenuated oral rotavirus
vaccine 116E in infants: a randomized, double-blind,
placebo-controlled trial. J Infect Dis. 2009;200:421-9.
62. Clements-Mann ML, Dudas R, Hoshino Y, Nehring
P, Sperber E, Wagner M, et al. Safety and immunogenicity of
live attenuated quadrivalent human-bovine (UK) reassortant rotavirus
vaccine administered with childhood vaccines to infants. Vaccine.
2001;19:4676-84.
63. Patriarca PA, Wright PF, John TJ. Factors
affecting the immunogenicity of oral poliovirus vaccine in
developing countries: review. Rev Infect Dis. 1991;13:926-39.
64. Tate JE, Patel MM, Steele AD, Gentsch JR,
Payne DC, Cortese MM, et al. Global impact of rotavirus
vaccines. Expert Rev Vaccines. 2010;9:395-407.
65. Moon KB. Differential rotavirus antibody
profiles in breast milk specimens from mothers with infants in
developed and developing countries. American Society for Virology
Conference Vancouver, BC, Canada. 2009 Oct.
66. Goveia MG, DiNubile MJ, Dallas MJ, Heaton PM,
Kuter BJ. Efficacy of pentavalent human-bovine (WC3) reassortant
rotavirus vaccine based on breastfeeding frequency. Pediatr Infect
Dis J. 2008;27:656-8.
67. Esposito DH, Tate JE, Kang G, Parashar UD.
Projected impact and cost-effectiveness of a rotavirus vaccination
program in India, 2008. Clin Infect Dis. 2011;52:171-7.
68. Rose J, Hawthorn RL, Watts B, Singer ME.
Public health impact and cost effectiveness of mass vaccination with
live attenuated human rotavirus vaccine (RIX4414) in India: model
based analysis. BMJ. 2009;339:b3653.
69. Press Release - Bharat Biotech announces the
price of Rotavac. Bharat Biotech. June 6, 2011.
http://www.bharatbiotech.com/BBIL-GAVI-ROTAVAC-Press%20Release-06June2011-F.pdf.
|

