Coronavirus disease 2019 (COVID-19) has been
declared as a pandemic, given its global spread. Children
account for 1-5% patients and are less likely to become severely
ill compared to adults; though, preschool children and infants
might have severe clinical features [1,2].
In March, 2020, the Indian Society of Pediatric
Nephrology (ISPN) decided to formulate guidelines on managing
children with renal diseases during the COVID-19 pandemic. A
writing committee and advisory board was formed to draft
guidelines, based on policies and guidelines from Ministry of
Health and Family Welfare, Indian Society of Nephrology and
international professional organizations, and evidence from
systematic and narrative reviews, trials and other reports.
Draft guidelines underwent multiple iterations before being
finalized.
Are Patients With Kidney Disease At Risk for COVID-19 and Poor Outcomes?
Co-morbidities associated with mortality during COVID-19 are
common in adult patients with chronic kidney disease (CKD), and
those on maintenance dialysis. Children with CKD especially
stage 4-5, those on hemodialysis (HD) or receiving
immunosuppressive agents are considered immunocompromised.
Patients with advanced CKD are malnourished and undergo
maintenance HD in busy units, increasing the risk of infection.
Analysis of confirmed patients with COVID-19 reported to the
Center for Disease Control (USA) revealed that patients with CKD
were 11 to 14-times more likely to be hospitalized and require
intensive care, respectively compared to those without CKD [3].
Reports from China suggest a less severe course of
the disease in dialysis compared to transplant recipients. At a
dialysis center in Renmin Hospital, Wuhan, 37 of 230 patients on
HD and 4 of 33 dialysis staff showed
severe acute respiratory syndrome coronavirus-2
(SARS-CoV-2) infection over 4-weeks [4]. Seven patients died, of
which 6 had COVID-19. The incidence of COVID-19 in HD patients
reported from China is similar to that from a similar cohort in
Italy. Of 20 adult transplant recipients in Brescia (Lombardy,
Italy) with COVID-19, 5 died, 4 were critically ill and 3
recovered. Similarly, among 21 HD patients with COVID-19; 5 died
while 4 recovered [5]. In a report on 15 adult transplant
recipients with COVID-19 from a single center in US, one-fourth
were ventilated with one death, while 50% were discharged [6].
In contrast, another report from US reported a higher mortality
among 36 adult kidney-transplant recipients with Covid-19
compared to general population as well as to patients
more than 70 years old with COVOD-19 (28% vs 5%
and 8-15%, respectively) [7].
However, none of the transplanted or dialyzed children
were infected in Lombardy that reported more than 8000 patients
with COVID-19 [personal communication: G. Montini, Pediatric
Nephrology Unit, Milano].
Experts feel that there is evidence that adult
patients with CKD, especially those on dialysis, transplant
recipients or receiving immunosuppressive therapy, are at
increased risk for SARS-CoV-2 infection, with significant
morbidity and unsatisfactory outcomes.
Recommendations for Patients and their Caregivers
Caregivers refer to parents/guardians taking care of health and
personnel needs of children. Patients with CKD, those on
immunosuppressive medications and transplant recipients, and
their caregivers should follow appropriate advice to reduce the
risk of getting sick. These measures include self-isolating and
staying at home to minimize contact between people; avoiding
non-essential travel, crowded places and large gatherings;
washing hands frequently with soap and water; and adopting cough
etiquette. Patients and caregivers should wear a triple-layer
mask while visiting healthcare facility including dialysis
units. In outpatient clinic, social distancing measures should
be strictly followed along with other measure of personal
protection. All used disposable gloves and masks should be
placed in a lined container before disposing them with other
household waste and wash hands with soap and water/alcohol-based
hand rub.
Caregivers should ensure around 4 weeks stock of
medications at home. They should contact their treating
physician or hospital, by phone or email, if child has fever,
cough, shortness of breath, with or without rhinorrhea, and
muscle aches or chills.
Healthcare Personnel
Health care personnel (HCP) refer to those directly related to
provision of health care services. HCP should receive
information about COVID-19, and training on institutional and
national protocols for evaluation and management [8]. Doctors,
dialysis nurses and technicians must follow guidelines for
prevention and control of infections and adhere to protocols for
identifying and reporting patients of COVID-19. Clinical
management of patients with COVID-19 is evolving, and doctors
are advised to stay updated.
Teleconsultations with patients and their families
are encouraged to minimize hospital visits. Simple strategies
are employed to support mental well-being of children and their
families, and mitigate anxiety and stress [9]. All staff members
in the dialysis unit should be trained in donning and doffing of
PPE [10].
Doctors, dialysis nurses and technicians should
stagger their schedule to reduce exposure to infection, and have
a reserve force that could be deployed for management of
patients [11]. We endorse recommendations of the National
Taskforce for hydroxychloroquine (HCQ) prophylaxis, for HCP
involved in care of suspected or confirmed patients with
COVID-19, and household contacts of laboratory confirmed cases
[12]. However, there is a need to be cautious and avoid its
indiscriminate use due to potential cardiac and other
toxicities. This practice may change as more evidence emerges on
benefits and safety of its use. HCQ should not be used for
prophylaxis in children younger than 15 year and those with
glucose-6-phosphate dehydrogenase deficiency. Caregivers and the
patient should be informed about the rationale of therapy,
contraindications and adverse effects.
Children Receiving Immunosuppressive Therapy
Immunosuppression and anti-proteinuric measures are cornerstones
of treatment in renal diseases. Immunosuppression was one of the
most common underlying conditions in a report on 345 children
with COVID-19 [2]. Patients receiving therapy with the following
agents should be considered immuno-compromised: Corticosteroids
(prednisolone, methylpre-dnisolone, dexamethasone): Prednisolone
dose >20 mg daily for >4-weeks in the last 6-months,or >5 mg
daily for >4 weeks with one or more immunosuppressive agents in
last 6-months; Calcineurin inhibitors (tacrolimus,
cyclosporine); Mycophenolate mofetil, azathioprine;
Cyclophosphamide: any dose (oral or intravenous) within the last
6 months; Rituximab: any dose within the last 6 months; and,
Plasma exchange in the preceding 6-weeks [13].
General Management
We advise that dose of immunosuppressive medication should not
be changed, since the risk of disease flare is higher than the
threat posed by COVID-19 in children. Patients should be advised
to keep ~4-weeks stock of immunosuppressive medications.
Hospital visits for non-emergency purposes are avoided and HCP
contacted through telecommunication. The physician may consider
deferring maintenance doses of IV cyclophosphamide or rituximab
in patients in sustained remission, and low risk of relapse on
case to case basis. Patients should be encouraged to maintain
hydration.
Nephrotic Syndrome, Glomerulonephritis, Vasculitis
We recommend that the first episode and relapse of nephrotic
syndrome should be treated promptly with standard dose of
prednisolone, as under normal circumstances. Delayed initiation
of therapy might result in complications associated with
anasarca and bacterial infections. Continuation of therapy may
be discussed telephonically. No changes should be made in
ongoing treatment of frequent relapsing and steroid resistant
nephrotic syndrome.
Decisions regarding initiating immunosuppressive
therapy in newly diagnosed patients with other glomerular
diseases or vasculitis, especially those from hotspots/ clusters
should be based on disease severity, renal histology and serum
creatinine, severity of proteinuria and co-morbidities, and
balancing the risk versus benefit of therapy [14]. We advise
initiating immunosuppression in newly diagnosed patients with
glomerular diseases or vasculitis, according to existing
guidelines, except in children with asymptomatic or low-grade
proteinuria and normal renal function. These patients may be
initially managed with salt restriction, and blood pressure
control using an angiotensin converting enzyme inhibitor or
angiotensin receptor blocker (ACE-I/ARB). As with nephrotic
syndrome, no changes are advised in ongoing or proposed
immunosuppressive therapy for patients with other glomerular
disorders or vasculitis.
Angiotensin Converting Enzyme Inhibitors
(ACE-I) and Angiotensin Receptor Blockers (ARB)
SARS-CoV-2, binds to its receptor, ACE-2 for entry into target
cells suggesting an increased ability of the virus to enter the
lungs in patients on ACE-I or ARB [15]. While there is debate
regarding safety of inhibitors of renin-angiotensin-aldosterone
axis, few studies have evaluated their effects on severity of
illness or mortality in COVID-19. Thus, therapy with ACE-I or
ARBs should be continued [16]. Abrupt discontinuation of
medications may be associated with uncontrolled hypertension,
and its consequences.
Hydroxychloroquine (HCQ)
Therapy with HCQ should continue in patients who are already
receiving the medication, e.g., for systemic lupus, vasculitis.
Risk of depleting stock of HCQ needs to be considered and
adequate stocks of medication should be ensured.
Renal Biopsy
It is suggested to prioritize patients with indications for
renal biopsy during the pandemic. Biopsies that are necessary
and have therapeutic implications, e.g., suspected allograft
rejection, rapidly progressive glomerulonephritis, small vessel
vasculitis and interstitial nephritis should be performed while
those for less emergent indications, e.g., steroid resistant
nephrotic syndrome, calcineurin toxicity and persistent
hematuria may be delayed.
Patients on Immunosuppression With Suspected or Confirmed COVID-19
Children on immunosuppression may present with mild symptoms but
have high risk of deterioration and require hospitalization.
Early identification of severe pneumonia and severe acute
respiratory illness (SARI) is important as it allows prompt
admission to a designated hospital ward or intensive care unit,
and initiation of treatment. Patient with suspected COVID-19
should be shifted to an isolation facility or designated COVID
area as soon as possible.
An approach to management of a child on
immunosuppressive medications with respiratory symptoms is
summarized in Fig. 1. While there is no specific
guidance on precise modification of immunosuppression, it seems
prudent to reduce or withhold immunosuppressive medications,
except stress doses of steroids in patients with severe COVID-19
requiring admission to intensive care units.
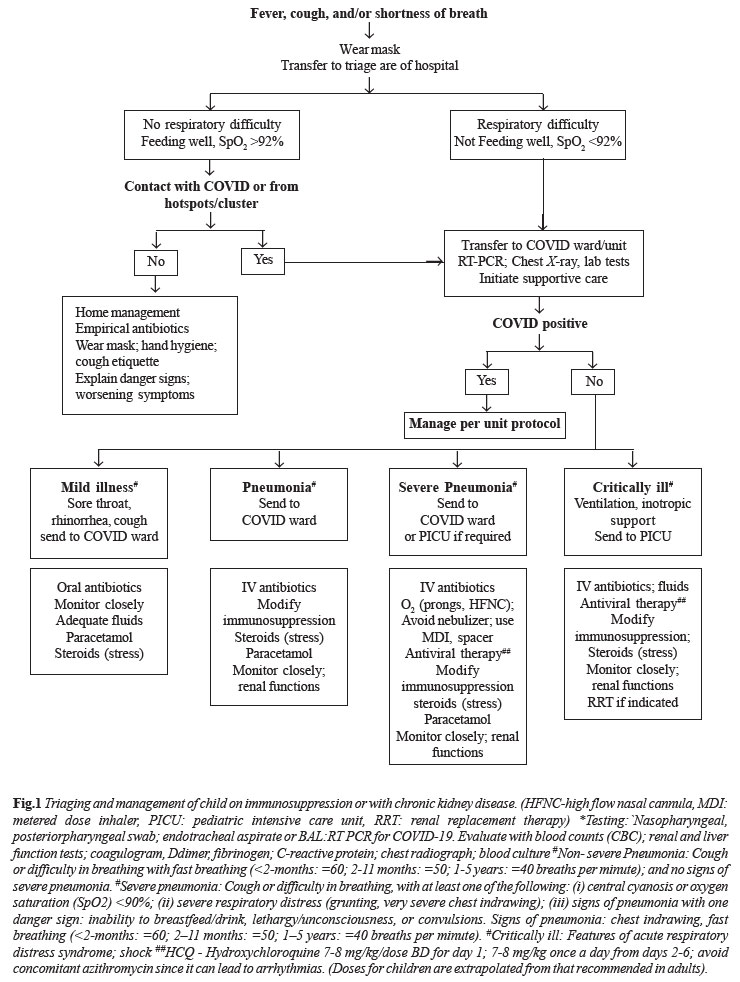 |
Given the lack of specific treatment, most patients
with COVID-19 require supportive care alone. More than 500
trials evaluating 150 drugs are being conducted worldwide [17].
Consistent with national guidelines, we suggest use of HCQ (7-8
mg/kg/dose twice daily for day 1, and 7-8 mg/kg once a day from
2-6 days for >12-yr) in patients with severe disease and
requiring ICU care [18]. The doses should be reduced by 50% for
children in CKD stage 5 and those on dialysis. Caregivers should
be informed about the rationale of therapy, and potential
adverse effects, especially prolonged QTc interval. There is
insufficient data to recommend the use of remdesivir,
lopinavir/ritonavir or other HIV protease inhibitors for
patients.
Children with Chronic Kidney Disease
Since children with CKD stage 3-5 are considered vulnerable to
infection with coronavirus, it is vital that children and
caregivers follow above mentioned precautions. Patients and
caregivers should maintain contact with their physicians,
especially for symptoms of COVID-19 including fever and
worsening respiratory symptoms. Paracetamol is safe for children
with fever, but treatment with other non-steroidal
anti-inflammatory drugs
(ibuprofen, naproxen) should be avoided.
Patients should continue taking antihypertensive
medications, targeting systolic and diastolic blood pressures to
~90th percentile
for age, gender and height. We recommend continued therapy with
ACE-I or ARB in patients with CKD who are receiving such
therapy. Abrupt withdrawal of these agents might result in
clinical instability and adverse outcomes.
Children on chronic ambulatory peritoneal dialysis
(CAPD) should continue sessions at home following the standard
protocol and precautions, avoid hospital visits, and maintain
adequate stock of fluids and consumables. Automated PD (APD)
machine should be disinfected using 70% alcohol-based solution
before and after each treatment. They should keep in contact
with the doctor or dialysis nurse, and inform promptly for
fever, symptoms of COVID-19 and peritonitis.
If COVID-19 is suspected in CKD, then patient
should be shifted to an isolation facility if available or to
designated COVID hospital as soon as possible and managed as per
standard guidelines (Fig. 1).
Hemodialysis (HD) Units
Inpatient and outpatient pediatric dialysis facilities must be
prepared for patients infected with SARS-CoV-2. The Ministry of
Health and Family Welfare has prepared comprehensive guidelines
for HD of COVID-19 patients [19]. The statement below is adapted
from the above guidance, specifically addressing needs for
children.
Children require HD in two situations: (i)
maintenance HD for end stage renal disease,(ii) dialysis
for acute kidney injury (AKI), related or unrelated to COVID-19.
An outbreak of COVID 19 in a dialysis facility is
defined as two or more COVID-19 infections resulting from a
common exposure, that is either suspected or
laboratory-confirmed as SARS-CoV-2 [20]. After identification,
the outbreak should be reported to the hospital authorities and
appropriate measures should be taken as per Government of India
guidelines [8].
General Recommendations
Patients on HD are advised not to postpone their dialysis
schedule. Phone numbers and contact information of the dialysis
unit should be provided to the patients.
Administrators need to ensure availability of
consumables, including dialysate, dialyzers and tubing,
catheters, fistula needles, disinfectants and medications.
It is necessary to educate HCP, patients and caregivers
about COVID-19, including hand hygiene, respiratory hygiene and
cough etiquette, use of facemasks and disposal of contaminated
items. Posters and literature (in local language) should be
available.
All dialysis personnel should use appropriate PPE, as per
institutional policy.
Dialysis waiting area, beds and nursing station(s) should
be equipped with alcohol-based sanitizers along with paper
napkins and foot operated plastic lined waste disposal bins.
Duties of HCP should be organized as per institutional
policy, with an overall aim to maintain a pool of reserve staff.
Bed side rounds by group of staff, group-studies and
office case-discussions involving teams should be minimized.
Patients with features suggestive of respiratory
infections (fever, cough) should be identified before they
enter the dialysis area. Caregivers are instructed to call the
unit to report fever or respiratory symptoms, so that they are
directed to an appropriate triage in the hospital. At each
dialysis visit, a staff member must perform a structured
interview for patient and caregivers, asking for: history of
fever, cough, respiratory difficulty and exposure to a patient
with COVID-19.
Children should be advised to use a triple-layer facemask
while in the waiting area, during dialysis and until they reach
home. Seats in the waiting area should be separated by at least
1 meter. To avoid overcrowding, children should be accompanied
by only one attendant who should also wear a facemask.
Dialysis patients, who have a parent or family member on
14-days quarantine, should continue to receive HD during this
period. Once the family members or caregiver are confirmed
SARS-CoV-2 positive, the dialysis patient should be isolated and
instructed to call the unit before arriving for
subsequent HD sessions, and to report fever or respiratory
symptoms.
Dialysis Unit: During Dialysis and Disinfection
If feasible, dialysis beds should be spaced at a minimum
distance of 2 meters.
Patients are instructed to wash their hands and fistula
arm before starting dialysis. Puncture sites should be cleaned,
and appropriately disinfected.
Disposable gloves should be used when handling laundry
from infected patients. Dirty laundry should not be shaken to
minimize the possibility of dispersing virus through air.
Bed linen should be changed between shifts, and used
linen placed in dedicated containers.
Disposable gowns must be discarded after use. Cloth gowns
are soaked in 1% hypochlorite solution for 20 minutes before
sluicing, and transported to laundry [19].
All surfaces and equipment in the unit should be cleaned
and disinfected at least once daily, and after each patient
shift. This includes bedside tables and lockers, dialysis
machines, patient monitors, syringe pumps, sphygmomanometers,
doorknobs, light-switches, counter tops, handles, desks, phones,
keyboards, toilets, faucets and sinks. For surfaces such as
carpeted floor, rugs and drapes, visible contamination is
removed, followed by appropriate cleaners indicated for these
surfaces. After cleaning, items should be laundered in
accordance with manufacturer instructions and dried completely.
Disinfection is done with either 1% bleach solution or
70% alcohol-based solution. Bleach is preferred for surfaces
that do not soak up water (example: floor). Use of 70% alcohol
based solution is recommended for disinfection of metallic
surfaces like door knobs or handles
Dialyzing
Patients With Suspected or Confirmed COVID-19
Most pediatric hemodialysis
units in developing countries are small, comprising 3- 6 beds.
In order to prevent transmission of infection, it is advised
that patients with suspected or confirmed COVID-19 be dialyzed
in a separate room, with separate access and with the door
closed. If a separate room is not available, the suspected
patient may be dialyzed in a corner or end-of-row station,
maintaining at least 2-meters separation in all directions,
preferably in the last shift of the day. The patient, as well as
other patients should wear 3 layered masks while dialysis
personnel, should wear appropriate PPE throughout the procedure.
Units that do not have enough space and/or dedicated work force
for dialyzing suspected or confirmed COVID-19 patients should
facilitate their transfer to a designated adult or pediatric HD
units until the testing is negative. This plan should be
communicated to the caregivers, which will help them prepare
accordingly.
It is recommended to use separate equipment, including
stethoscopes, thermometers, saturation probes, and blood
pressure cuffs, with cleaning and disinfection between shifts.
Stethoscopes are disinfected with alcohol-based solutions.
Dialysis personnel should not touch the patient or use
stethoscopes, unless necessary.
Surfaces and equipments located within 1-meter of the
patient should be disinfected, as detailed previously. All
disposable supplies are discarded.
Dialysis personnel taking care of a patient with
suspected COVID-19 should not look after other patients during
the same shift. Staff should self-report symptoms of fever,
cough or breathlessness.
Institutional and national guidelines should be followed
for managing patients with suspected or confirmed COVID (Fig.
2).
Personal Protective Equipment (PPE) for Dialysis Personnel
Dialysis personnel shall be instructed regarding the need
for personal protection. They will be trained for donning and
doffing of PPE, its proper use and disposal [10].
We suggest the use of triple-layer masks, head cover,
gloves, water-impermeable gown and shoe-covers for HCP working
in the unit. Personnel involved in procedures involving aerosol
generation, venepuncture and dialysis access should follow
standard contact and droplet precautions, and should wear N95
mask and disposable face-shield [21]. Table I
summarizes the protective equipment required for different
levels of anticipated contact.
Further, in children requiring plasmapheresis using HD
machine and in the HD unit, all measures suggested in the
hemodialysis section should be followed.
Dialyzing Children With COVID-19 and Acute Kidney Injury
The incidence of AKI in patients with COVID-19 ranges from 6-15%
[22]. Patients with CKD on maintenance dialysis may require care
in an intensive care unit. A proportion of patients with
secondary bacterial infection will have septic shock, drug
nephrotoxicity or worsening of existing CKD, severe enough to
require renal replacement therapy (RRT).
The goal is to deliver RRT in a safe and timely
manner. Children may need to be dialyzed in shared spaces with
adults, if dedicated space is not available. Centres should
anticipate surge in COVID-19 related AKI and the need for
dialysis may outpace available facilities. As in any patient
with AKI, indications for initiating RRT and choice of modality
i.e., peritoneal dialysis, HD, continuous renal
replacement therapy (CRRT), and sustained low efficiency
dialysis (SLED), is based on resources and expertise, and
patient hemodynamic status. We suggest the following:
Access: Central venous catheter for HD or PD catheter
should be placed with complete PPE. For patients who already
have arteriovenous fistula, CRRT and SLED may be considered
provided monitoring for potential complications of the procedure
is possible [23].
Prescribing CRRT: In centers having facility for CRRT, the
treatment time for continuous veno-venous hemodialfiltration may
be reduced to 10-12 hours in order to make the machine available
for a greater number of patients. In case of shortage of
replacement fluids, the dose could be reduced to 1000ml/m2 instead
of 2000 ml/m2, especially after first
few-hour and once metabolic control is achieved. Normal saline
may also be used as replacement fluid.
Anticoagulation: Centers dialyzing patients with COVID-19 have
reported circuit clotting in CRRT and SLED if anticoagulants are
not used. Anticoagulation is done, as per unit protocol.
In case of non-availability of pumps for heparin, low
molecular weight heparin may be used (enoxaparin single dose
0.5-1 mg/kg; dalteparin<15-kg: 1500 IU; 15-30 kg: 2500 IU) [24].
In order to minimize exposure, the CRRT or HD
machine may be set-up outside the patient area, and then taken
into the room and connected.
After treatment, all equipment should be
disinfected with 1% sodium hypochlorite before being removed
from the room.
Transport of patients with suspected or confirmed
COVID-19 to a central dialysis unit is not recommended. These
patients should be dialyzed bedside, using portable reverse
osmosis.
Acute peritoneal dialysis should be considered when
hemodialysis machines are not available. An automated cycler
should be used to minimize patient contact. The drain fluid is
disposed, are per protocol. All consumables like tubings,
dialyzers and replacement solutions bags should be discarded.
Transplant Recipients
Kidney transplant recipients must be considered highly
susceptible to SARS-CoV-2 infection. Data on COVID-19 in
transplant patients is however limited. It has been observed
that among adults renal transplant recipients with COVID-19, 60%
require hospitalization and 25 -30 % require ICU care with
mortality rate of 5% [6,25]. However, a recent study has shown
high early mortality in transplant
recipients than general population with Covid 19
infection [7].
General Precautions
Transplant recipients are advised to follow general precautions
for patients with CKD.
Movement outside the home, including for follow up
hospital visits, should be restricted. Teleconsultation may be
utilized to contact HCP.
When outside the house, transplant recipients and
caregivers should use triple-layer mask and prevent touching of
nose and mouth.
It is essential to maintain a 4-weeks stock of
medications. If the family is unable to obtain medications, the
transplant team should be informed.
Transplantation During COVID-19 Pandemic
Unlike other solid organs, kidney transplantation is performed
in a relatively stable patient, receiving maintenance HD.
Transplantation is associated with marked immunosuppression,
which might not be in patients interest during the pandemic.
Transplant recipients may also require respiratory support and
ICU monitoring during the peri-operative period, facilities that
are scarce during the outbreak. Using these facilities for an
elective procedure might also reduce their availability for a
critically ill COVID-19 patient. We believe that the risks of
performing kidney transplantation outweigh the benefits to
either the patient or the healthcare system. We recommend
postponing live-related donor transplants until the outbreak has
abated.
The National Organ and Tissue Transplant
Organization (NOTTO) has advised temporary suspension of
deceased and live related transplant program [26]. However, if
pandemic lasts for longer duration, then reconsideration of
recommendation is advised.
Transplant Recipients With COVID-19
Transplant recipients presenting with cough or shortness of
breath with or without fever, history of contact with known
patient, or with features of SARI should be screened for
SARS-CoV-2 infection by RT-PCR of nasopharyngeal swabs. These
patients may have atypical features such as coryza, diarrhea and
fatigue. Fever is reported in 50-87%, while diarrhea and
lymphopenia are observed in 30% and 50% patients, respectively
[6,23]. One-third may have no radiographic findings. A high
index of suspicion is necessary to diagnose COVID-19 in
transplant recipients.
Supportive management for transplant recipients
with COVID-19 is shown in Fig. 3. For patients
with mild disease, reduction of immunosuppression is not
recommended as this might result in allograft rejection. In
sicker patients (with pneumonia, but not critically ill), the
anti-proliferative agent (mycophenolate or azathioprine) should
be discontinued. The dose of prednisolone is increased to 0.5-1
mg/kg when therapy with mycopheno-late is stopped. Dose of
calcineurin inhibitors (CNI) is reduced to target lower levels
(Tacrolimus adjusted to achieve a trough of 4-6 ng/mL;
Cyclosporine 100 -150 ng/mL). In critically ill children
(requiring ventilation and inotropic support), CNI may be
reduced further or discontinued [27,28]. Such patients are
managed with steroid monotherapy, at a higher dose. Once
recovery begins, immune-suppressants should be reintroduced and
increased to pre-illness doses 14-days after two nasopharyngeal
swabs are negative.
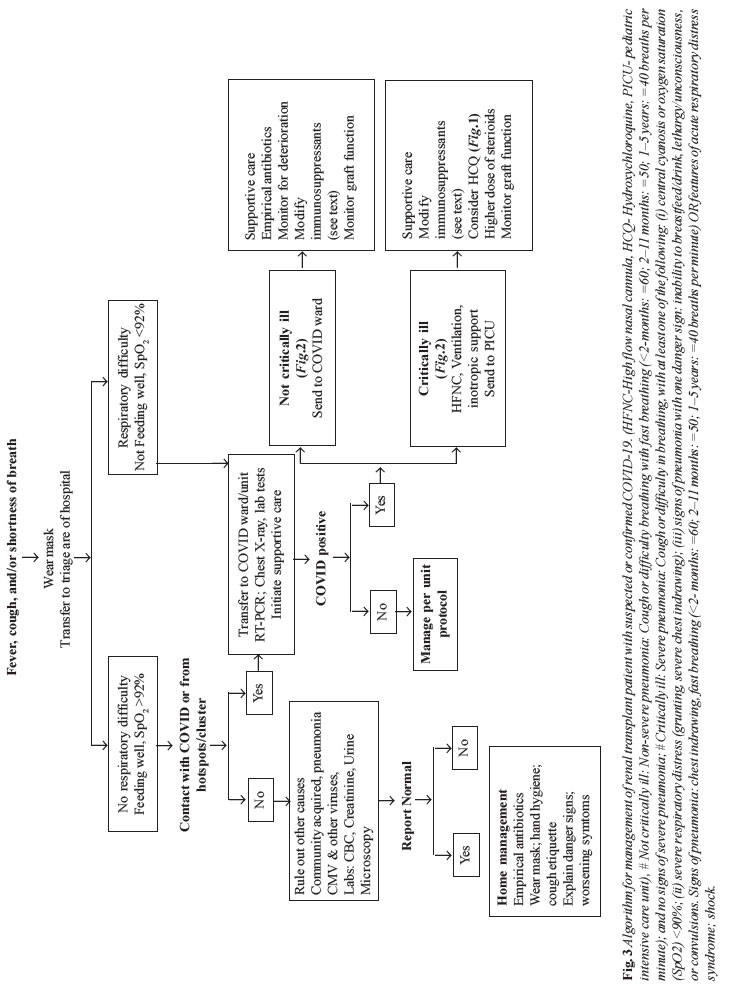 |
There is no evidence to support the use of
antiviral treatment for COVID-19. Drugs being examined include
lopinavir/ritonavir, remdesivir, favipiravir, HCQ, tocilizumab,
interferon-a and intravenous
immuno-globulins. Interaction of medications with CNI and
sirolimus needs to be considered. Lopinavir/ritonavir,
darunavir/ritonavir, chloroquine and HCQ can potentially
increase CNI levels, while tocilizumab decreases CNI and
sirolimus levels [29]. Other causes for fever, including
bacterial or viral infections should be ruled out. Antibiotics
should be used for empiric treatment of bacterial infections and
modified based on culture sensitivity results.
CONCLUSIONS
The present guidelines of the Indian Society of Pediatric
Nephrology on managing patients with kidney diseases during the
COVID-19 pandemic are based on current literature and expert
views. While children constitute a small proportion of patients
with COVID-19, those with chronic disorders constitute a
high-risk group and at-risk for adverse outcomes. Therapeutic
guidelines are likely to change as evidence emerges from large
case series and randomized controlled trials.
REFERENCES
1. Dong Y, Mo
X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of
COVID-19 among children in China. Pediatrics. 2020 Mar 16. [Epub
ahead of print]. Available from:
https://pediatrics.aappublications.org/content/early/2020/03/16/peds.2020-0702.1.
Accessed on May 14, 2020.
2. CDC
COVID-19 Response Team. Coronavirus Disease 2019 in Children-
United States, February 12April 2, 2020. MMWR Morb Mortal Wkly
Rep. 2020; 69:422-26.
3. Ministry of
Health and Family Welfare, Government of India. COVID-19 INDIA.
Available from: https://www.mohfw.gov.in/. Accessed on
May 8, 2020.
4. Ma Y, Diao
B, Xifeng L, Zhu J, Liang W, Liu L, et al. 2019 novel
coronavirus disease in hemodialysis patients: Report from one HD
center in Wuhan, China. Available from:
https://www.medrxiv.org/content/10.1101/2020.02.24. 20027201v2/.
Accessed on April 12, 2020.
5. Alberici F,
Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, et al.
A single center observational study of the clinical
characteristics and short-term outcome of 20 kidney transplant
patients admitted for SARS-CoV2 pneumonia.
Kidney Int. 2020 Apr 9. [Epub ahead of print]. Available
from: https://www.kidney-international.
org/article/S0085-2538(20)30365-3/fulltext. Accessed on May
14, 2020
6. Columbia
University Kidney Transplant Program.
Early Description of Coronavirus 2019 Disease in Kidney
Transplant Recipients in New York.
J Am Soc Nephrol. 2020 Apr 21. [Epub ahead of print].
Available from https://jasn.asnjournals.org/content/early/
2020/04/27/ASN.2020030375.long. Accessed on May 14, 2020.
7. Akalin E,
Azzi Y, Bartash R,
Seethamraju H, Parides M, Hemmige V, et al.
Covid 19 and kidney transplan-tation. N Engl J Med. 2020
Apr 24 [Epub ahead
of print]. Available from:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7200055/pdf/NEJMc2011117.pdf.
Accessed on May 14, 2020.
8. Ministry of
Health and Family Welfare Directorate General of Health Services
(EMR division). Revised guidelines on clinical management of
COVID-19. Available from:
www.mohfw.gov.in/pdf/RevisedNationalClinical
ManagementGuidelineforCOVID1931032020.pdf/. Accessed on
April 26, 2020.
9. Board of
Governors in Supersession of the Medical Council of India.
Telemedicine Practice Guidelines. Available from:
https://www.mohfw.gov.in/pdf/Telemedicine.pdf/. Accessed on
April 26, 2020.
10. Ministry
of Health and Family Welfare Directorate General of Health
Services (EMR). Guidelines on rational use of personal
protective equipment. Available from:
www.mohfw.gov.in/pdf/Guidelinesonrationaluseof
PersonalProtectiveEquipment.pdf/. Accessed on April 26,
2020.
11. Ministry
of Health and Family Welfare Directorate General of Health
Services (EMR). SOP for reallocation of residents/PG students
and nursing students as part of hospital management of COVID.
Available from:
https://www.mohfw.gov.in/pdf/COVID19SOPfordoctorsand nurses. pdf/.
Accessed on April 26, 2020.
12. Indian
Council of Medical Research. Recommendation for empiric use of
hydroxychloroquine for prophylaxis of SARS-CoV-2 infection.
Available from:
https://icmr.nic.in/sites/default/files/upload_documents/HCQ_Recommen
dation_22March_final_MM_V2.pdf/. Accessed on April 26, 2020.
13. Wallace D,
on behalf of BAPN. Information and guidance for children on
hemodialysis, peritoneal dialysis and immune suppression
(including renal transplants). Available from:
https://renal.org/covid-19/paediatric-useful-information-resources/.
Accessed on April 26, 2020.
14. For
patients with CKD using immunosuppressive therapy. Available
from:
https://renal.org/stratified-risk-prolonged-self-isolation-adults-children-receiving-immuno
suppression-disease-native-kidneys/. Accessed on April 25,
2020.
15. Liu Z,
Xiao X, Wei X, Li J, Yang J, Tan H, et al. Composition
and divergence of coronavirus spike proteins and host ACE2
receptors predict potential intermediate hosts of SARSCoV-2.
J Med Virol. 2020 Feb 26. [Epub ahead of print].
Available from: https://onlinelibrary.
wiley.com/doi/full/10.1002/jmv.25726.
Accessed on May 14, 2020
16.
Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA,
Solomon SD. Renin-angiotensin-aldosterone system inhibitors in
patients with Covid-19.
N Engl J Med. 2020;382:1653-59.
17. Covid-19
clinical trials tracker. Available from:
https://covidclinicaltrials.com. Accessed on April 26, 2020.
18. Indian
Council of Medical Research. Testing Labs Details. Available
from:
https://icmr.nic.in/sites/default/files/upload_documents/Strategey_for_COVID19_Test_v4_
09042020.pdf /. Accessed on April 07, 2020.
19. Ministry
of Health and Family Welfare. Revised Guidelines for Dialysis of
COVID 19 patients.
Available from:
https://www.mohfw.gov.in/pdf/RevisedGuidelinesfor
DialysisofCOVID19Patients.pdf.
Accessed on April 26, 2020.
20.
Schwierzeck V, Kφnig JC, Kόhn J, Mellmann A, Correa-Martνnez CL,
Omran H, et al.
First reported nosocomial outbreak of severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric
dialysis unit. Clin Infect Dis. 2020 Apr 27. [Epub ahead of
print]. Available from:
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa491/5825509.
Accessed on May 14, 2020.
21. Shen Q,
Wang M, Che R, Li Q, Zhou J, Wang F, et al; Chinese
Society of Pediatric Nephrology and Chinese Medical Doctor
Association of Pediatric Nephrology. Consensus recommendations
for the care of children receiving chronic dialysis in
association with the COVID-19 epidemic. Pediatr Nephrol. 2020
Apr 24. [Epub ahead
of print]. Available from:
https://link.springer.com/article/10.1007/s00467-020-04555-x.
Accessed on May 14, 2020.
22. Naicker S,
Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V. The novel Coronavirus
2019 epidemic and kidneys.
Kidney Int. 2020; 97: 824-28.
23. Al Rifai
A, Sukul N, Wonnacott R, Heung M. Safety of arteriovenous
fistulae and grafts for continuous renal replacement therapy:
Michigan experience. Hemodial Int. 2018;22 :50-55.
24. Davenport
A. Alternatives to standard unfractionated heparin for pediatric
hemodialysis treatments. Pediatr Nephrol 2012; 27:1869-79.
25. Hiremath
S, Topf JM. Transplant. Available from:
www.nephjc.com/news/covidtx/. Accessed on April 07, 2020.
26. National
transplant specific guidance for COVID-19. Available from:
https://notto.gov.in/WriteReadData/Portal/News/711_1_FINAL_GUIDANCE_COVID-19_
31.03.2020.pdf /. Accessed on April 22, 2020.
27. Guidance
on the management of transplant recipients diagnosed with or
suspected of having COVID19. Available from:
https://bts.org.uk/wpcontent/uploads/2020/03/Clinical_management_transplant_recipients.pdf/.
Accessed on April 07, 2020.
28. DESCARTES
expert opinion regarding the management of immunosuppressive
medication for kidney transplant patients during the COVID-19
pandemic. Available from:
https://www.era-edta.org/en/wp-content/uploads/2020/04/Expert-opinion-on-ISD-in-Covid-19.pdf
/. Accessed on April 07, 2020.
29.
Characteristics of potential antiviral agents under evaluation
for treatment of COVID-19. Available from:
https://www.covid19treatmentguidelines.nih.gov/thera
peutic-options-under-investigation/. Accessed on April 26,
2020.

