Typhoid
(enteric) fever is a major public health disorder worldwide
including India [1]. Several typhoid conjugate vaccines (TCVs)
in which Vi capsular polysaccharide of Salmonella typhi
is conjugated to the various carrier proteins have been
developed to overcome the immunological drawbacks of the
conventional typhoid polysaccharide vaccines [2].
Two such TCVs containing tetanus toxoid as the carrier
protein have already been approved and marketed in India viz,
Tybar-TCV (Bharat Biotech International Ltd.) [3] and Pedatyph
(Bio-Med Pvt. Ltd.) [4]. The former contains 25 mcg of the Vi
polysaccharide while the latter contains only 5 mcg of the Vi
polysaccharide. TCVs can also be administered to infants and
toddlers. The current study was conducted to evaluate the
immunogenicity and safety of a new investigational indigenously
developed TCV (Test TCV) in the target population.
METHODS
This was a pre-licensure, randomized, multicentre,
single-blind, non-inferiority, phase II/III clinical study
conducted at 8 centres (tertiary care or multispecialty
hospitals) during June to November, 2016.
The study was approved by the Regulatory Authority and
the respective Institutional Ethics Committees of all the study
sites. The study was registered prospectively with Clinical
Trial Registry of India.
Prior to screening, a written informed consent with a prior
consent for audio-video recording of the consent process was
obtained from the adult subjects and guardians of the pediatric
subjects; an assent was also obtained from the pediatric
subjects aged
³7 years. Healthy subjects of either gender aged
6 months to 45 years were considered eligible if the adult
subjects or guardians of the pediatric subjects were literate
enough to fill the adverse event (AE) details in the diary
cards. The subjects were excluded if they had a history of
hypersensitivity to any component of the vaccine, typhoid fever
or vaccination against typhoid fever within the past 3 years,
fever or infectious disorder of any origin of >3 days in the
past month, any vaccination within the past 7 days; any febrile
illness (³37.5oC)
at the time of enrollment; any clinically significant systemic
disorder, immunological disorder, coagulation disorder or
thrombocytopenia; any anticoagulant, immuno-suppressive or
immunostimulant therapy; administered blood, blood products or
immunoglobulins within the past 3 months or planned
administration during the study; pregnant and lactating women
and female subjects not using acceptable contraceptive measures;
participation in another clinical trial in the past 3 months; or
history of alcohol or drug abuse in the past one year. Urine
pregnancy test was done for adult females during the screening.
The subjects were equally divided in the adult (18-45 years) and
the pediatric (6 months to <18 years) cohorts; the enrolment in
the pediatric cohort commenced after review of day 21 safety
data of all the subjects enrolled in the adult cohort by an
independent data and safety monitoring board.
Pediatric cohort was stratified according to age into 6
month to less than 2 year, 2 to less than 5 year and 5 to less
than 18 year.
A centralized block randomization plan of block size four was
generated from www.randomization.com and a unique
sequence of randomization numbers from this plan was provided to
each study site. Eligible subjects were randomized (1:1) to
receive a 0.5 mL single-dose of either the test TCV (Cadilla
Healthcare Ltd., Ahmedabad, India) (Batch No. BO09S03) or the
comparator TCV (Batch No. 76DL15026) which contained 25 mcg
purified Vi capsular polysaccharide of S. typhi
conjugated to tetanus toxoid. As the antigenic composition of
the test TCV mimics that of Typbar-TCV, it was selected as the
comparator (Comparator TCV). Comparator TCV has also been
prequalified by the World Health Organization (WHO) in December,
2017 and it is indicated for active immunization against S.
typhi infection in 6 months to 45 years age group [5].
Comparator TCV was procured from the market for this study. The
vaccine was administered in the upper arm or in the
anterolateral aspect of the upper thigh for younger children, at
baseline (day 0) following which the subjects were closely
observed for at least 30 minutes for any immediate AEs. Loading
of the injection for vaccination was done out of sight of the
subjects/guardians to maintain single-blinding. The subjects
were later followed up on an outpatient basis on day 7±3, 21±7
and 42+14.
Diary cards were given to the adult subjects or the guardians of
the pediatric subjects to record solicited local (pain, redness,
swelling and induration) and systemic (fever, headache, nausea,
vomiting, malaise, arthralgia and myalgia) AEs for 7 days
post-vaccination and unsolicited AEs till the end of the study.
Any abnormality in the vitals or physical examination was also
to be reported as an AE. The severity of AEs was graded as mild,
moderate or severe as per the defined criteria (supplementary
table) and causality was assessed as per the WHO’s criteria for
AEs following immunization [6]. In addition, the investigators
also graded the tolerability to the vaccine based on the
reported AEs.
Two mL blood samples were collected at baseline and 6 weeks
post-vaccination for assessment of anti-Vi IgG antibody titre by
the commercial Vacczyme ELISA kits (Binding Site Group Ltd., UK)
at the central accredited laboratory. The primary outcome was
seroconversion rate which was defined as four-fold
or higher rise in anti-Vi antibody titre post-vaccination
as per the WHO recommendations [7,8]. The secondary outcomes
were geometric mean titre (GMT) of antibodies and seroconversion
rate and GMT of antibodies in both age cohorts. The safety
variables were local or systemic AEs, serious AEs (SAEs)
reported, if any, and overall tolerability evaluation by the
investigators based on the reported AEs as follows: Excellent -
no AE, Good - mild AE(s), Fair - moderate AE(s) and Poor -
severe or serious AE(s).
Assuming the seroconversion rate of at least 95% based on the
published results of the comparator vaccine [9], a sample size
of 238 subjects (1:1 allocation) was calculated to demonstrate
the non-inferiority of the test TCV as compared to the
comparator TCV considering 90% power, 2.5% level of significance
and dropout rate of 15%.
Statistical analysis: The test TCV was
considered non-inferior to the comparator TCV if the lower limit
of 95% CI for the
difference between their seroconversion rates was above the
pre-defined non-inferiority limit of -10% [8]. The GMTs between
the groups were compared using the unpaired t-test while
the GMTs within the groups were compared using the paired t-test
after log transformation of antibody titres. The seroconversion
rate and the incidence of AEs was compared using Chi-square or
Fisher’s exact test.
Immunogenicity was assessed for both per-protocol and modified
intention-to-treat analysis (defined as all randomized subjects
who completed the study including the subjects with protocol
violations) while all the vaccinated subjects were considered
for the safety assessment.
RESULTS
In this study, 240 subjects (120 pediatric, 123 females) were
randomized (Fig. I). The mean (SD) age, height,
weight and body mass index of the subjects were 16.1 (12.5)
years, 130.2 (35.8) cm, 37.1 (22.9) kg and 19.0 (4.8) kg/m2 respectively. The baseline
characteristics of the subjects are as mentioned in Table
I.
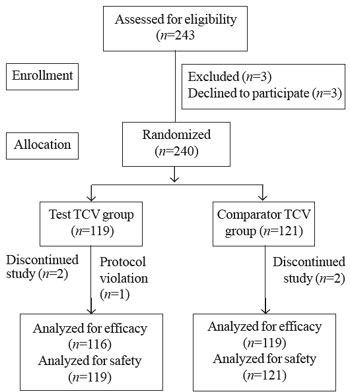 |
| Fig. 1. Study flow
chart. |
The seroconversion rates for the
overall population, and the adult and the pediatric cohorts were
94.8%, 96.6% and 93.1% in the test group and 91.6%, 91.7% and
91.5% in the comparator group, respectively. The difference
between proportions (95% CI) were 3.2% (-3.2%, 9.7%), 4.9%
(-3.5%, 13.3%) and 1.6% (-8.1%, 11.2%) for the overall
population, and the adult and the pediatric cohorts,
respectively. The seroconversion rates for the age groups of 6 m
to <2 y, 2 to <5 y and 5 to <18 y were 100%, 90% and 90% in the
test group and 81.8%, 100% and 94.4% in the comparator group
respectively; (P>0.5).
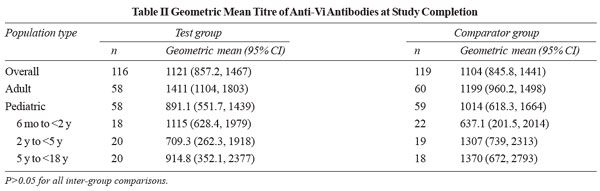
A significant rise in the GMTs of anti-Vi antibodies was
reported post-vaccination in both the study groups and in
various age groups (P<0.0001). The GMTs of antibodies at
the end of the study were comparable between the groups (P>0.05)
(Table II). The results of seroconversion and GMTs
of antibodies were similar when analyzed by modified
intention-to-treat analysis (data not shown).
 |
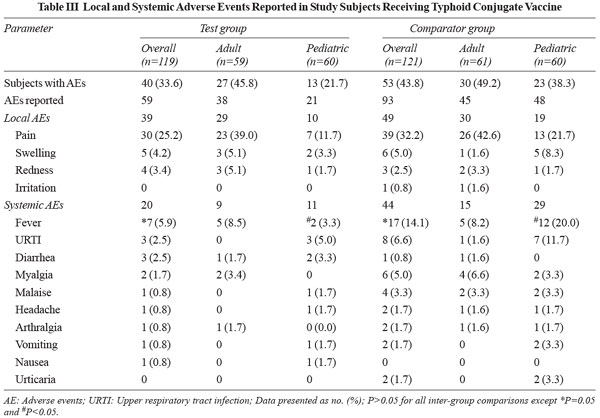 |
In this study, 33.6% and 43.8% subjects in the test and
comparator group respectively had reported AEs (P=0.11).
The characteristics of AEs reported in the overall population
and the adult and the pediatric cohorts are given in Table
III. All the AEs
resolved within 7 days of their occurrence with 91.5% AEs in the
test group and 93.6% AEs in the comparator group getting
resolved within 3 days of occurrence. There was no SAE reported
during the study in either of the two study groups. Most of the
AEs were mild in intensity, 93.2% in the test group and 81.7% in
the comparator group.
A certain, probable or possible association of AEs with
the vaccine was seen in 88.1% and 85% of test and comparator
group respectively.
The overall tolerability assessment was excellent, good, fair
and poor in 66.4%, 31.1%, 2.5% and 0% subjects in the test
group, and 56.2%, 33.9%, 9.1% and 0.8% subjects in the
comparator group, respectively (P=0.09).
DISCUSSION
In the present study, seroconversion rate post-vaccination
with the test TCV was non-inferior to the comparator TCV, and
GMT of antibodies post-vaccination were comparable for both the
vaccines. The safety of the test TCV evaluated in terms of
reported AEs was also comparable to the comparator TCV.
The seroconversion rate with the
comparator TCV reported earlier varied from 91.9-100% in various
age groups [9,10]. The seroconversion rates reported with
Pedatyph were 83% [11] and 100% [12] in pediatric subjects. The
seroconversion rates with varying concentrations of another TCV,
Vi-CRM197 (1.25 mcg, 5 mcg, 12.5
mcg and 25 mcg) in adult subjects in phase I and II studies were
also in the range of 95-100% [13]. The seroconversion rate
reported for the test TCV in this study was also similar.
Likewise, the GMT of antibodies post-vaccination in this study
also correlated well with that reported for the comparator TCV
in previous studies in which similar antibody assessment method
was used [9,10]; however, the GMT of antibodies post-vaccination
with other TCVs could not be directly compared due to the
difference in the antibody assessment method [11-16]. Further,
the safety data reported in this study is also consistent with
that reported for other TCVs [13,17] and typhoid polysaccharide
vaccines [18-20].
The limitations included
single-blind nature of the study as the difference in the
physical characteristics and packaging of the test TCV and the
comparator TCV precluded double-blinding.
The study was conducted in a limited sample size (albeit
sufficient to draw statistical conclusions) with a short-term
follow-up. However, a long-term extension of this study is being
conducted in which the persistence of antibodies around 3 years
after primary vaccination will be evaluated.
Owing to the improved immunological
properties, permission for use in younger children including
infants and longevity of the immune response of TCV, WHO has
recommended a single-dose of TCV from 6 months to 45 years of
age in endemic regions to prevent typhoid fever [21]. The
results of this study indicate that the immunogenicity and
safety of the test TCV is comparable to that of the comparator
TCV.
Contributors:
RK, AKK, UN, SKJ, TRB, RV, SS, VKG: study conduct, medical care
of the study participants and data acquisition; PD,RM: study
concept and design, overall study coordination, data analysis
and interpretation; PP: study concept and design and manufacture
of the test vaccine. All authors had full access to clinical
trial data. PD, RM: prepared the manuscript and other authors
provided their feedback for revising it for the intellectual
content. All authors have approved the final version of this
manuscript. All authors agree with the interpretation of data
and its representation in the manuscript.
Funding:
M/s. Cadila Healthcare Ltd., Ahmedabad, India.
Competing interests:
RK, AKK, UN, SKJ, TRB, RV, SS,VKG: were the clinical trial
investigators and they received honorarium from the sponsor for
the conduct of the study. PD,PP,RM: are employees of M/s. Cadila
Healthcare Ltd.
| |
|
WHAT IS ALREADY
KNOWN?
-
Typhoid conjugate vaccines are
associated with a better immunological response as
compared to the conventional unconjugated polysaccharide
vaccines.
WHAT THIS STUDY
ADDS?
|
References
1. Thamizhmani R,
Bhattacharya D, Sayi DS, Bhattacharjee H, Muruganandam N, Ghosal
SR, et al. Emergence of fluoroquinolone resistance in
Salmonella enterica serovar Typhi in Andaman and Nicobar
Islands, India. Indian J Med Res. 2012;136:98-101.
2. Szu SC. Development of
Vi conjugate - a new generation of typhoid vaccine. Expert Rev
Vaccines. 2013;12:1273-86.
3. Bharat Biotech
International Ltd. Typbar TCV™. Available from:
https://www.bharatbiotech.com/images/typbartcv/Typbar-TCV-Package-Insert.pdf.
Accessed August 19, 2019.
4. Bio-Med Private Limited.
PedaTyph™. Available from:
http://www.biomed.co.in/peda-typh/. Accessed August 19,
2019.
5. World Health
Organization. WHO Prequalified Vaccines. Last updated: July 19,
2019. Available from: https://extra-net.who.int/gavi/PQ_Web/.
Accessed August 19, 2019.
6. World Health
Organization. Adverse Events Following Immunization (AEFI):
Causality Assessment. Available from:
http://apps.who.int/iris/bitstream/10665/191391/1/a87773_eng.pdf.
Accessed August 19, 2019.
7. World Health
Organization. Annex 3 Guidelines on the quality, safety and
efficacy of typhoid conjugate vaccines. WHO Technical Report
Series No. 987, 2014. Available from:
http://www.who.int/biologicals/areas/vaccines/ TRS_987_
Annex3.pdf. Accessed August 19, 2019
8. World Health
Organization. Annex 1 Guidelines on clinical evaluation of
vaccines: regulatory expectations. WHO Technical Report Series
No. 924, 2004. Available from:http://www.who.int/biologicals/publications/trs/areas/vaccines/clinical_evaluation/035-101.pdf.
Accessed August 19, 2019.
9. Mohan VK, Varanasi V,
Singh A, Pasetti MF, Levine MM, Venkatesan R, et al.
Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid
conjugate vaccine (Typbar-TCV) in healthy infants, children, and
adults in typhoid endemic areas: a multicenter, 2-cohort,
open-label, double-blind, randomized controlled phase 3 study.
Clin Infect Dis. 2015;61:393-402.
10. Jin C, Gibani MM, Moore
M, Juel HB, Jones E, Meiring J, et al. Efficacy and
immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the
prevention of typhoid fever using a controlled human infection
model of Salmonella Typhi: A randomised controlled, phase 2b
trial. Lancet. 2017;390:2472-80.
11. Chinnasami B,
Mangayarkarasi V, Prema A, Sadasivam K, Davis MJ. Safety and
immunogenicity of salmonella typhi Vi conjugate vaccine (Peda
Typh™) in children upto five years. Int J Sci Res Publ.
2013;3:1-5.
12. Mitra M, Shah N, Ghosh
A, Chatterjee S, Kaur I, Bhattacharya N, et al. Efficacy
and safety of vi-tetanus toxoid conjugated typhoid vaccine
(PedaTyph™) in Indian children: School based cluster randomized
study. Hum Vaccin Immunother. 2016;12:939-45.
13. van Damme P, Kafeja F,
Anemona A, Basile V, Hilbert AK, De Coster I, et al.
Safety, immunogenicity and dose ranging of a new Vi-CRM 197
conjugate vaccine against typhoid fever: randomized clinical
testing in healthy adults. PLoS One. 2011;6:e25398.
14. Chinnasami B, Sadasivam
K, Vivekanandhan A, Arunachalam P, Pasupathy S. A study on
longevity of immune response after vaccination with Salmonella
typhi Vi conjugate vaccine (Pedatyph™) in children. J Clin Diagn
Res. 2015;9:SC01-3.
15. Canh DG, Lin FY, Thiem
VD, Trach DD, Trong ND, Mao ND, et al. Effect of dosage
on immunogenicity of a Vi conjugate vaccine injected twice into
2- to 5-year-old Vietnamese children. Infect Immun.
2004;72:6586-8.
16. Lin FY, Ho VA, Khiem
HB, Trach DD, Bay PV, Thanh TC, et al. The efficacy of a
Salmonella typhi Vi conjugate vaccine in two-to-five-year-old
children. N Engl J Med. 2001;344:1263-9
17. Bhutta ZA, Capeding MR,
Bavdekar A, Marchetti E,
Ariff S, Soofi SB, et al. Immunogenicity and
safety of
the Vi-CRM197 conjugate
vaccine against typhoid fever
in adults, children, and infants in south and southeast
Asia: Results from two randomised, observer-blind, age
de-escalation, phase 2 trials. Lancet Infect Dis.
2014;14:119-29.
18. Miyazu M, Kikuchi H,
Hamada A, Fukushima S, Ouchi K, Bosch Castells V, et al.
A Japanese study to assess immunogenicity and safety of a
typhoid Vi polysaccharide vaccine. Vaccine. 2015;33:6697-702.
19. Sanofi Pasteur Inc.
Typhoid Vi polysaccharide vaccine typhim Vi®. Available from
https://www.fda.gov/media/75993/download. Accessed August
19, 2019.
20. Sanofi Pasteur Limited.
Typhim Vi®. Available from https://pdf.hres.ca/dpd_pm/00048782.PDF.
Accessed August 19, 2019.
21. World Health
Organization. Typhoid vaccines: WHO position paper, March 2018 -
Recommendations. Vaccine. 2019;37:214-6.

