F
lexible fiberoptic bronchoscopy
(FFB) and bronchoalveolar lavage (BAL) have emerged as important
diagnostic tools for the evaluation and treatment of children with lung
and airway problems. The rigid bronchoscope is made of a metal body and
can be passed through the tracheobronchial tree under general
anesthesia. The major advantages of FFB as compared to rigid
bronchoscopy include smaller external diameter of the new pediatric
flexible scopes, the ability to change direction (flex and extend within
the airway), fine illumination with fiberoptic technology and airway
dynamics evaluation. Flexible bronchoscopy was introduced by Ikeda in
1968 [1]. Pioneering work by Wood and Fink popularized the use of
flexible bronchoscopy in pediatrics [2]. In recent years, smaller models
of flexible bronchoscope have become available thereby creating
opportunities for applications that had hitherto been unthinkable.
Visualization of airways by FFB has now become an integral part in the
management of infants, neonates and children. Technological advances in
the field of fiberoptics and cameras have opened new horizons in the
field. Higher magnification and digital cameras have further enhanced
the utility of FFB in neonates and children.
The Equipment
The necessary equipment consists of a flexible
bronchoscope of an appropriate size and a light source. Photographic
equipment such as a still or video camera is highly desirable.
Additionally, intravenous equipment, drugs used for anaesthesia and
resuscitation, suction apparatus, oxygen delivery system and appropriate
specimen collection containers are needed. Appropriate sized mask,
endotracheal tube, laryngoscope and resuscitation bag should be easily
available. It is highly desirable that advanced airway management
equipments should be checked and documented to be in functional
condition by the person responsible for sedation and monitoring during
the procedure. The most important consideration is selecting the
appropriate size of bronchoscope in children owing to their narrow
airways. As the patient has to breathe around the flexible bronchoscope,
the size of the bronchoscope should not be more than two-third of the
diameter of the trachea (Table I). The smallest sized
bronchoscope available should be used in neonates, infants and young
children to reduce obstruction of the airway lumen by the bronchoscope
during the procedure (which would impair ventilation) and to minimize
local mucosal trauma [3]. Thus, for neonates flexible bronchoscopes with
outer diameter (OD) of 2.8 mm, for infants and young children 3.6 mm OD
scopes and for older children bronchoscope of 4.8 mm OD are used.
Ultrathin bronchoscope (2.2 mm OD) are used in neonates weighing less
than one kg and during intraoperative airway assessment. Fig.
1 shows a pediatric flexible bronchovideoscope with placement of
working channel and light source at the tip of scope and equipments
required to obtain diagnostic material during bronchoscopy. The quality
of images obtained and visualization increases with the increase in
diameter (and hence the number of fiberoptic cables) of the flexible
bronchoscope.
Table I Specifications of Pediatric Flexible Bronchoscopes
|
Outer Diameter(mm) |
Working Chamber (mm) |
Suction Channel (mm) |
Biopsy |
Brush |
ET tube |
Utility |
|
4.8 |
2.2 |
2.0 |
Good |
+ |
>5.5 |
7-8 y or >20 y |
|
3.6 |
1.2 |
1.2 |
Small |
+ |
>5 |
Standard pediatric |
|
2.8 |
1.2 |
1.2 |
Small |
+ |
>4 |
Newborn-infants-children |
|
2.2 |
- |
No |
No |
- |
>3 |
Newborn and infants <6 mo |
|
1.8 |
- |
No |
No |
- |
|
|
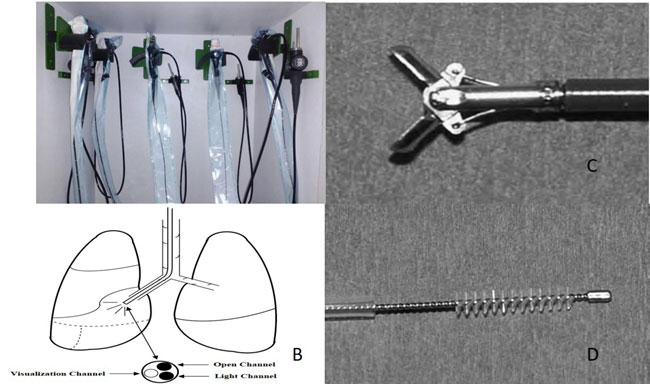 |
|
Fig. 1 A: Pediatric fiberoptic
bronchoscopes; B: Placement of different channels in a flexible
bronchovideoscope; C: Tip of endobronchial biopsy forceps; D:
Cytology brush tip shown outside its sheath.
|
Setting
The place where the bronchoscopy is conducted is
dependent on the clinical condition of the patient, the technical and
clinical abilities of the bronchoscopist and the nature of the available
equipment and personnel. The procedure can be safely performed in a
bronchoscopy suite, at the bedside in the intensive care unit or in the
operating room. The team also consists of a technician trained in
organizing the procedure, cleaning and handling the equipment and a
trained nurse to prepare drugs required during procedure. A person
qualified to provide sedation, monitoring and resuscitation during the
procedure should support the bronchoscopist. It is recommended that one
person observes and monitors the patient and another helps administer
medications and assist the bronchoscopist as needed.
Pre-procedure Evaluation
The detailed history-taking and physical examination
should be performed before performing FFB. History of obstructive sleep
apnea, previous anaesthetic difficulties or previous head and neck
surgery should also be taken. Radiographic studies should be available
for review during the bronchoscopy. The procedure is simple, well
tolerated and generally requires few hours (four) of hospitalization.
Prior informed consent for sedation and FFB procedure should be
obtained. The child should not ingest water four hours prior to
procedure or any solid food six hours prior to procedure. The various
prerequisites for FFB are enlisted in Box 1.
|
Box 1 Pre-requisites for Flexible
Fiberoptic Bronchoscopy
• Indication for flexible bronchoscopy
• Appropriate size bronchoscope
• Facility and skill for bronchoscopy
• Facility for monitoring during bronchoscopy
• Facility and skills for cardiopulmonary
resuscitation
• Informed consent from the parents/guardians
• Microbiology and cytopathology to analyze
the bronchoalveolar lavage fluid
• Facility for video-recording the bronchoscopy (preferable).
|
Established guidelines and manufacturer’s
recommendations for inspection, maintenance, storage, cleaning, and
manual or automated reprocessing of flexible bronchoscopes, should be
strictly followed. During bronchoscopy staff should wear protective
clothing (gowns or plastic aprons, masks/visors, and gloves). Universal
precautions are recommended for all staff that may be exposed to body
secretions [3].
Indications
FFB helps obtain anatomical and dynamic information
of the airways and to perform cytological and microbiological studies.
The indications include symptoms or radiological anomalies that cannot
be explained by non-invasive methods or to obtain samples from the lower
airways [4]. The various indications for flexible bronchoscopy are
highlighted in Box 2.
|
Box 2 Indications of Flexible Fiberoptic
Bronchoscopy in Children
Evaluation of airways
• Suspicion of a foreign
body
• Persistent stridor
• Persistent wheezing
• Hemoptysis
• Persistent or recurring
pneumonia
• Localized pulmonary
hyperlucency
• Persistent or recurring
atelectasis
• Problems related with the
artificial airways
• Miscellaneous (large
burns, phonatory anomalies)
Obtaining cultures (bronchoalveolar
lavage, bronchial biopsy)
• Pneumonia in
immunosuppressed patients
• Chronic interstitial
pneumonia
• Pneumonitis due to
hypersensitivity
• Pulmonary hemosiderosis
• Eosinophilic pneumonia
• Other (sarcoidosis,
alveolar proteinosis, histiocytosis)
• Endoluminal obstructive
pathology
• Aspiration lung syndromes
Therapeutic indications
• Difficult or selective
intubations
• Aspiration of
endobronchial secretions
• Instillation of medication
•Management of the
foreign body combined with rigid bronchoscope
|
Contra-indications
Flexible bronchoscopy is generally well tolerated.
Appropriate measures to optimize the patient’s condition should be taken
to minimize risk. The indication for FFB should be individualized. The
procedure should only be performed when the benefits outweigh the risks.
The absolute contraindications that impede performing bronchoscopy are
severe refractory hypoxemia, hemodynamic instability, uncorrected
hemorrhagic diathesis and the lack of authorization for the procedure by
the parent or guardian. The relative contraindications depend on the
experience of the team and the level of critical care in the hospital.
In very premature newborns and children with congenital cyanotic
cardiomyopathies with an increase in bronchial collateral circulation,
severe pulmonary hypertension or coagulation alterations, risk-benefit
assessment must be done.
Sedation and Monitoring
The aim of sedation in a child is to ensure that the
patient is safe, comfortable and reasonably still during the procedure
while maintaining adequate oxygenation and ventilation. Bronchoscopy can
be performed in sedation with benzodiazepines or narcotics or under
general anaesthesia. ‘Conscious sedation’, where patient can follow
verbal instructions and reflexes are preserved, is not recommended [4].
An anaesthesiologist or intensivist must be present to administer drugs
and monitor the patient continuously during the procedure. Spontaneous
respiration is preferred during diagnostic procedures, hence level of
sedation should be appropriate. Diagnosing dynamic airway anomalies may
be difficult in deeply sedated child with no spontaneous respiration.
Use of positive pressure ventilation during the procedure helps in
maintaining adequate ventilation in diagnostic procedures. Preferred
drugs for local anaesthesia are 2% lidocaine jelly for the nose and 1%
lidocaine spray for the pharynx and larynx. For analgesia and sedation,
midazolam (0.05-0.2 mg/kg), fentanyl (1-3 mcg/kg) or ketamine (1-3
mg/kg) are used.
Monitoring of the patient includes continuous
evaluation of heart rate, respiratory rate, color, head position and
assessment of gas exchange by continuous pulse oximetry. Continuous ECG
and invasive monitoring are desirable in sick critical patients like
children with cardiac diseases. A sedated patient should not be left
alone or unobserved. The pediatric patient must be adequately monitored
until awake. Supplemental oxygen should be maintained after the
procedure until patient has recovered from sedation and adequate
oxygenation on room air is documented. When the procedure is performed
on an outpatient, the child should be tolerating oral intake prior to
discharge.
Route and Procedure
Flexible bronchoscopy is usually performed
trans-nasally and can be performed orally or via artificial
airway. Oxygen mask or nasal prongs can be used simultaneously while
using the nasal route or the oral route. Nasal route can be employed
while the patient is on high flow nasal cannula ventilation. Special
noninvasive ventilation facemasks (interfaces) are available that can be
used for FFB while patient continues to receive positive pressure
ventilation. Oral route is used when nasal route is not feasible as in
choanal atresia, nasal bleeding or trauma. Laryngeal mask airway can be
used for ventilation through oral route. Endotracheal tubes and
tracheostomy tubes of minimum size of 4 mm are used in critically ill
patients with an appropriate size bronchoscope [5].
The operator stands or sits at the head of the
patient with the gurney in low position in order to avoid the equipment
from getting curved. Forced curves in the scope can damage its fibers
and make it difficult to handle. An appropriate sized bronchoscope is
chosen according to the child’s age. The anatomy and the functionality
of the pharyngeal and laryngeal structures (sublingual glands, tonsils,
arytenoids, epiglottis and vocal cords) are studied if the access is
nasal. Further progression through the larynx is done by centering the
end of the bronchoscope in the angle of the anterior corner of the vocal
cords, introducing it by means of posterior flexion when the patient
inhales. In order to make the passage easier and to prevent the
appearance of laryngeal spasm, a local dose of 1% lidocaine can be
sprayed through the working channel. After reaching the subglottic
space, lidocaine spray and proceed method is used to suppress cough
while negotiating trachea and bronchi. The bronchoscope is further
advanced until wedged in a desired sub-segmental bronchus at the desired
location.
Bronchoalveolar lavage: BAL is usually
carried out in the most-affected area (identified radiologically and/or
endoscopically). The right lower lobe offers better fluid recovery and
is the preferred site for BAL in diffuse lung disease. In infants it is
often easier to perform BAL in the right lower lobe. If BAL and
trans-bronchial biopsies are planned in the same patient, BAL should be
performed first. BAL is carried out using normal sterile saline
previously warmed to body temperature. Limited information exists on the
amount of fluid and the number of aliquots that should be used in order
to obtain samples representative for the alveolar compartment in
children of different ages and sizes. The amount of fluid instilled can
be determined using body weight as 3 mL/kg of normal saline divided into
three equal fractions for children weighing <20 kg and 3 mL/kg in 20 mL
portions in children weighing >20 kg [5]. The aliquots are instilled
using a syringe via the suction channel of the FFB and then
gentle suction (50-80 mmHg) is applied to collect the lavage specimen in
the collection trap [6]. The minimum amount of BAL fluid necessary to
perform the typical battery of laboratory tests depends on the clinical
scenario and endoscopic findings. For adults it is recommended that the
minimal total volume retrieved is >30% of the instilled volume [3]. The
BAL fluid can be sent for investigations and can aid in diagnoses (Box
3). Centers for Disease Control/National Health Safety Network
criteria for ventilator-associated pneumonia (VAP) with common bacterial
organisms specifies diagnostic threshold values as >10
4
colony-forming units (cfu)/mL from BAL and >103
cfu/mL from protected specimen brushing [7]. BAL results have been used
as a reference test for the diagnosis of VAP [8]
|
Box 3 Possible Investigations in
Bronchoalveolar Lavage Fluid
Cell count and differential
• Alveolar macrophages
(Normal >80%)
• Neutrophils (Normal <3%)
• Eosinophilia (Normal
<1-2%)
• Lymphocytosis (Normal
<15%)
• Erythrocytes
Microbiology
• Cultures
• Stains and
Immunohistochemistry : Gram stain; KOH stain; Periodic
acid-Schiff (PAS); Direct fluorescent stain (DF) or
Ziehl-Neelson (ZN stain); Modified acid fast stain (Kinyoun);
Giemsa stain; Inclusion bodies
• Polymerase chain reaction
(PCR): Mycobacteria tuberculosis and numerous pathogens.
• Quantitative or
semiquantitative cultures: particularly for ventilator
associated pneumonia.
• Diagnostic of infection if
organism identified: Pneumocystis carinii, Strongyloides etc.
Cytology
• Foamy macrophages
• Malignancies
• Sulfur granules
• Hemosiderin-laden macrophages
• Langerhans cells: >5% suggestive of
Pulmonary Langerhans cell histiocytosis;
• Fat and Lipid stain (e.g. Sudan
III): Lipoid pneumonia (aspiration)
|
Diagnostic Utility
Flexible Bronchoscopy
Flexible bronchoscopy allows direct visualization of
the upper and lower airway, enabling detection of anatomical defects
that may not be visible on radiologic imaging. Presence of endobronchial
infections, granulations, tumors can be confirmed by FFB. Also, it
allows dynamic changes in the airway, and hence diagnosis of dynamic
airway obstructions like airway malacias and excessive dynamic airway
compression (EDAC) is possible only by FFB which may be missed on
virtual bronchoscopy obtained with computerized tomography of chest.
Flexible bronchoscopy along with dynamic CT (not very readily available)
is used to diagnose EDAC and differentiate it from tracheobronchomalacia
in adults [9]. Apart from anatomical delineation, FFB also helps in
collection of bronchoalveolar lavage and biopsies that can be subjected
to microbiological, pathological examination. Bronchoscopy can be
indicated in children with unusual presentations of chronic cough or
wheeze, and cystic fibrosis [10]. Few bronchoscopic diagnoses are shown
in Web Fig. 2. Bar-Zohar,
et al. [11] could detect airway pathologies in 69% of PICU patients
who underwent bronchoscopy for suspected airway malformations.
Endobronchial Ultrasound (EBUS)
Ultrasound probes (balloon-tipped, miniaturized) are
passed through the working channel of flexible bronchoscope to visualize
the tracheobronchial wall and immediate surrounding structures. It is
useful in lymphoma and mediastinal tumors where the extent of lymphnodes
can be determined, avoiding a CT scan. The probe is pressed against the
bronchial wall and balloon is inflated. A biopsy can be obtained from
the adjacent lymph node for diagnosis of tuberculosis, sarcoidosis,
malignancy etc. [12].
Therapeutic Procedures
Aspiration of secretions: FFB can be
useful for resolving atelectasis due to the retention of secretions or
mucus plugging. Repeated large volume BAL is the recommended treatment
in pulmonary alveolar proteinosis and in acquired conditions like lipoid
pneumonia [13,14].
Difficult and selective intubations:
Bronchoscopy can act as a guideline for intubation in cases of
craniofacial anomalies and multiple malformations syndromes [15]. It has
also been used for selective bronchial intubation and intubation in
special conditions like suspected or proven cervical spine injuries
[16].
Removal of foreign body: The removal of foreign
body using FFB is a complicated procedure in children. Some publications
endorse the good performance of FFB for foreign body removal [17].
Though FFB is the procedure of choice for diagnosis of foreign body,
rigid bronchoscopy remains the gold standard for its retrieval in
children and in adults [18]. In children, rigid bronchoscopy is
preferred as it offers the advantages of general anesthesia, assisted
ventilation, larger instruments and a greater variety of accessories.
The ideal procedure would be to initiate with FFB, which allows for
greater reach in the exploration and the identification of the foreign
body, extraction with rigid bronchoscope, and if required, a final
revision with FFB to rule out a residual foreign body.
Transbronchial biopsy: Transbronchial biopsy
(TBB) involves obtaining a biopsy sample of lymph nodes surrounding the
carina or of the pulmonary parenchyma for microscopic analysis (Fig.
1). The samples from lymph nodes helpin diagnosing conditions like
extra-pulmonary tuberculosis and lymphoma. The samples from pulmonary
parenchyma are preferred for diagnosis of rejection in children with
lung transplant. The size of the TBB sample obtained determines the
yield of TBB. Multiple small sized lymph node samples are better while
fewer larger samples may be required to accurately diagnose cancer and
ILDs [19].
Bronchial washing: Bronchial washing can be used
to study the bronchial mucosa or to culture bronchial secretions (Fig.
1). It is a useful technique for the diagnosis of ciliary dyskinesia,
tuberculosis and pneumonia in patients with mechanical ventilation and
lung infections in immunosuppressed patients.
Balloon dilatation: Balloon dilatation of
stenosed or narrow airways can be done by rigid bronchoscopy and FFB.
Ideal cases for balloon dilatation are web-like stenosis, benign
strictures, complication of endotracheal intubation or granulomatous
disease. It is a minimally invasive, safe and rapid procedure [20].
Pitfalls
There are situations where radiological imaging may
take precedence over FFB. Especially when there is a lack of expertise
or infrastructure required to ensure safety of the procedure. In cases
of persistent pneumonia with poor response to antibiotic treatment, BAL
can be obtained specifically from affected sites that are identified on
a CT scan., The diagnostic yield of FFB alone is low in peripheral lung
nodules in malignancies or infections (fungal or tubercular) [21]. In
congenital parenchymal lung malfor-mations, CECT is the investigation of
choice for diagnosis. FFB aids to identify associated airway anomalies
[22]. This helps in planning the type of surgery and need for airway
reconstruction, postoperative management [4].
Cleaning and Disinfection
Cleaning and disassembling of the flexible
bronchoscope is a delicate procedure because of the complex valves and
channels. Bronchoscopes need to be cleaned and disinfected thoroughly
after every use in a dedicated room to prevent cross-infection amongst
patients. The bronchoscopes must be checked for leaks to prevent leakage
of fluid into the optic system. After disassembling the parts, thorough
cleaning with tap water or detergent water is necessary. Preferred agent
for disinfection of cleaned bronchoscope is 2% glutaraldehyde. Broncho-scopes
should be immersed in this solution for 20 min to ensure killing of all
pathogens. Disinfection should be followed by rinsing with deionized
water to remove all the disinfectant from the bronchoscope. Gas
sterilization with ethylene oxide at temperature <55°C is safe but not
always practical. Conventional heat sterilization methods can destroy
the bronchoscope. Removable, heat-stable parts like suction valve etc
can be steam autoclaved. Damaged bronchoscopes should be gas sterilized
as they cannot be immersed in disinfectant [23].
Complications
Pediatric bronchoscopy is a generally well-tolerated
procedure. Bronchoscopy-related complications can be broadly classified
into (i) complications due to anesthesia (which account for 50%
of the complications); and (ii) complications due to bronchoscope
in the airway. Hypoxemia may occur during the procedure requiring
initiation of supplemental oxygen. Increased airway resistance,
excessive sedation, and disturbance of ventilation-perfusion
relationship can cause hypoxemia. In patients who are hemodynamically
unstable and on mechanical ventilation, FFB can negatively affect lung
compliance and airway resistance. Inadequate use of topical anesthesia
may result in adverse reactions such as laryngospasrn, bradycardia, or
other vagal nerve mediated phenomena. Inadequate sedation may lead to
patient discomfort. On the other hand, excessive use of sedation may
result in depression of respiration. Post- bronchoscopy fever may occur,
especially following bronchoalveolar lavage. Mechanical complications of
bronchoscopy may include epistaxis, pneumothorax, and hemoptysis.
Trans-bronchial biopsy is the most common cause of bronchoscopy related
hemoptysis. Rarely, infections complicating FFB are seen due to
procedural and disinfection lapses [23].
Conclusion
FFB in neonates, infants and children has many
diagnostic and therapeutic benefits. Bronchoalveolar lavage and lung
tissue obtained with FFB can aid in the diagnosis of many pulmonary
diseases. Dynamic airway conditions like laryngomalacia,
tracheobronchomalacia can be diagnosed and quantified accurately with
FFB under sedation. Proper pre-procedure preparation and monitoring
during and after FFB should be followed to minimize complications. All
neonatologists and pediatricians should be aware of the indications and
utilities of FFB in pediatric patients.
Contributors: Both authors contributed to
review of literature, manuscript writing and its approval, and are
accountable for all aspects related to the review.
Funding: None; Competing interest: None
stated.
References
1. Ikeda S. Recording of the endoscopic picture. J
Jap Med Instr.1967;37:291.
2. Wood RE, Sherman JM. Pediatric flexible
bronchoscopy. Ann Otol Rhinol Laryngol. 1980;89:414-6.
3. Faro A, Wood RE, Schechter MS, Leong AB,
Wittkugerl E, Abode K, et al. Official American Thoracic Society
technical standards: flexible airway endoscopy in children. Am J Respir
Crit Care Med. 2015;191:1066-80.
4. Wood RE, Boesch RP. Bronchoscopy and
bronchoalveolar lavage in pediatric patients. In: Wilmott RW,
Boat TF, Bush A, Chernick V, Deterding RR, Ratjen F, editors. Kendig and
Chernick’s disorders of the respiratory tract in children. 8th ed.
Philadelphia, PA: Elsevier Saunders; 2012. p.131-44.
5. Grigg J, van den Borre C, Malfroot A, Pierard D,
Wang D, Dab I. Bilateral fiberoptic bronchoalveolar lavage in acute
unilateral lobar pneumonia. J Pediatr. 1993;122:606-8.
6. De Blic. Flexible Bronchoscopy. In: Eber E,
Midulla F, editors. ERS Handbook of Pediatric Respiratory Medicine. 1st
ed. UK: Charlesworth Press; 2013. p.132-9.
7. Stokes DC, Shenep JL, Parham D, Bozeman PM,
MarienchekW, Mackert PW. Role of flexible bronchoscopy in the diagnosis
of pulmonary infiltrates in pediatric patients with cancer. J Pediatr.
1989;115:561-7.
8. Sachdev A, Chugh K, Sethi M, Gupta D, Wattal C,
Menon G. Diagnosis of ventilator-associated pneumonia in children in
resource-limited setting: A comparative study of bronchoscopic and
nonbronchoscopic methods. Pediatr Crit Care Med. 2010;11:258-66.
9. Murgu S, Stoy S. Excessive dynamic airway
collapse: A standalone cause of exertional dyspnea? Ann Am Thorac
Soc. 2016;13:1437-9.
10. Nicolai T .The role of rigid and flexible
bronchoscopy in children. Paediatr Respir Rev. 2011;12:190-5.
11. Bar-Zohar D, Sivan Y. The yield of flexible
fiberoptic bronchoscopy in pediatric intensive care patients. Chest.
2004;126:1353-9.
12. Bolliger CT, Mathur PN, Beamis JF, Becker HD, Cavaliere
S, Colt H, et al. ERS/ATS Statement on Interventional Pulmonology.
European Respiratory Society/American Thoracic Society. Eur Respir J.
2002;19:356-73.
13. Garg G, Sachdev A, Gupta D. Pulmonary alveolar
proteinosis. Indian Pediatr. 2009;46:521-3.
14. Sachdev A, Anand P, Gupta D. Lipoid pneumonia- An
unusual cause of acute respiratory distress syndrome. Indian Pediatr.
2015;52:63-4.
15. Finer NN. Flexible fiberoptic bronchoscopy. In:
Spitzer AR, editors. Intensive care of the fetus and neonate. Mosby,
St.Louis, 1996. p. 531-7.
16. Pandharikar N, Sachdev A, Gupta N, Gupta S, Gupta
D. Chest trauma: A case for single lung ventilation. Indian J Crit Care
Med. 2016;20:248-50.
17. Kapoor R, Chandra T, Mendpara H, Gupta R, Garg S.
Flexible bronchoscopic removal of foreign bodies from airway of
children: Single center experience over 12 years. Indian Pediatr.2019;
56:560-2.
18. Salih AM, Alfaki M, Alam-Elhuda DM. Airway
foreign bodies: A critical review for a common pediatric emergency.
World J Emerg Med. 2016;7:5-12.
19. Sehgal IS, Bal A, Dhooria S, Gupta N, Ram B,
Aggarwal AN, et.al. Predictors of successful yield of
transbronchial lung biopsy in patients with sarcoidosis. J Bronchology
Interv Pulmonol. 2018:25,31-6.
20. Ernst A, Silvestri GA, Johnstone D, American
College of Chest Physicians. Interventional Pulmonary Procedures:
Guidelines from the American College of Chest Physicians. Chest.
2003;123:1693.
21. De Roza MA, Quah KH, Tay CK, Toh W, Li H,
Kalyanasundaram G,
et al.
Diagnosis of periphera lung lesions via conventional flexible
bronchoscopy with multiplanar CT planning. Pulm Med. 2016;2016:5048961.
22. Sachdeva A, Chhawchharia R, Gupta D, Gupta N.
Flexible fiberopic bronchoscopy directed interventions in neonatal
intensive care unit. Indian Pediatr.2019;56:563-6.
23. Terkawi RS, Altirkawi KA, Terkawi AS, Mukhtar G,
Al-Shamrani A. Flexible bronchoscopy in children: Utility and
complications. Int J Pediatr Adolesc Med. 2016;3:18-27.

