|
|
|
Indian Pediatr 2019;56: 566-570 |
 |
Clinical Spectrum of Congenital Anomalies of
Kidney and Urinary Tract in Children
|
|
Bondada Hemanth Kumar 1,
Sriram Krishnamurthy1,
Venkatesh Chandrasekaran1,
Bibekanand Jindal2
and Ramesh Ananthakrishnan3
From Departments of 1Pediatrics, 2Pediatric
Surgery and 3Radiology, Jawaharlal Institute of Postgraduate
Medical Education and Research (JIPMER), Puducherry, India
Correspondence to: Dr Sriram Krishnamurthy,
Additional Professor, Department of Pediatrics, JIPMER,
Puducherry-605006, India.
Email: [email protected]
Received: October 02, 2018;
Initial review: January 04, 2019;
Accepted: May 14, 2019.
|
|
Objective: To evaluate the
clinical spectrum and patterns of clinical presentation in congenital
anomalies of kidney and urinary tract. Methods: We enrolled 307
consecutively presenting children with congenital anomalies of kidney
and urinary tract at the pediatric nephrology clinic. Patients were
evaluated clinically, with serum biochemistry, appropriate imaging and
radionuclide scans. Results: The most common anomaly was primary
vesicoureteric reflux (VUR) (87, 27.3%), followed by pelviureteral
junction obstruction (PUJO) (62,20.1%), multicystic dysplastic kidney
(51 16.6%), non-obstructive hydronephrosis (32, 10.4%) and posterior
urethral valves (PUV) (23, 7.4%). 247 (80.4%) anomalies had been
identified during the antenatal period. Another 33 (10.7%) were
diagnosed during evaluation of urinary tract infection, and 21 (6.8%)
during evaluation for hypertension at presentation. Obstructive
anomalies presented earlier than non-obstructive (7 (3, 22.5) vs
10 (4, 24) mo: (P=0.01)). The median (IQR) ages of presentation
for children with PUV (n=23), VUR (n=87) and PUJO (n=62)
were 4 (2, 14) mo, 10 (5, 27) mo, and 7 (3, 22.5) mo, respectively. Nine
(2.9%) children had extrarenal manifestations. Conclusions: The
median age at clinical presentation for various subgroups of anomalies
indicates delayed referral. We emphasize the need for prompt referral in
order to initiate appropriate therapeutic strategies in children with
congenital anomalies of kidney and urinary tract.
Keywords: CAKUT, Hydronephrosis, Multicystic
renal dysplasia, Vesico-ureteral reflux.
|
|
C
ongenital anomalies of the kidney and urinary
tract (CAKUT) are an important cause of morbidity in children, and
contribute significantly to end-stage renal disease (ESRD). About 30-60%
of ESRD in children are due to CAKUT [1-4]. CAKUT includes a wide
spectrum of anomalies such as pelviureteral junction obstruction (PUJO),
multicystic dysplastic kidney (MCDK), renal hypodysplasia, horse-shoe
kidney, ectopic kidney, primary vesicoureteric reflux (VUR), posterior
urethral valve (PUV), and vesicoureteral junctional obstruction (VUJO).
It is important to diagnose these anomalies and initiate therapy to
prevent or delay the onset of ESRD. We therefore, studied the clinical
presentation patterns in children with CAKUT. The primary objective of
this study was to evaluate the clinical spectrum and patterns of
clinical presentation in CAKUT. The secondary objectives were to study
the clinical characteristics of obstructive and non-obstructive CAKUT,
and to study the extrarenal manifestations in these patients.
Methods
This descriptive study was conducted at the Pediatric
Nephrology outpatient clinic of a tertiary hospital from December 2015
through September 2017 after obtaining approval from the Institute
Ethics Committee.
The study recruited consecutively presenting children
aged <13 years with CAKUT. Children with polycystic kidneys and
neurogenic bladder were excluded. Definitions of clinical entities –
acute kidney injury (AKI) [5], urinary tract infection (UTI) [6],
chronic kidney disease (CKD) [7] and hypertension [8] – were as per
standard guidelines.The diagnosis and management of antenatal
hydronephrosis was performed as per standard guidelines [9,10]. Weight
for age Z scores were calculated from WHO growth charts [11]. The
diagnostic criteria for various CAKUT were as follows:
(a) PUJO: PUJO was defined by an
obstructive pattern on ethylenecysteamine (EC) diuretic renography
i.e., a curve that rises continuously over 20 minutes or
plateaus, despite furosemide administration and post-micturition
[10].
(b) PUV: The diagnosis was
established by a micturating cystourethrography (MCU) showing
dilated or elongated prominent posterior urethra; and confirmed by
cystoscopy.
(c) Primary VUR: MCU was performed
for confirming the diagnosis of primary VUR, and then classified
into grades I to V [12]. Secondary VUR was excluded by presence of
bladder anomalies/ureterocele.
(d) MCDK: It was diagnosed by
unilateral multiple cysts of varying sizes on ultrasound, with
altered echoes without cortico-medullary differentiation; and a DMSA
renal scan showing minimal/ no function.
(e) Other anomalies such as renal
hypoplasia/dysplasia, horseshoe kidney, crossed renal ectopia,
duplex-collecting system were diagnosed on renal ultrasonogram.
Clinical and laboratory data were recorded in a cross
sectional manner in a pre-designed structured proforma.
Statistical analyses: Normally distributed data
was compared by Student’s t-test, non-normally distributed data by
Mann-Whitney U test, and proportions by chi-square test/ Fisher’s exact
test. SPSS 23.0 software (SPSS Inc. Chicago, Illinois) was used for
analysis.
Results
A total of 307 children with CAKUT were enrolled (Fig.
1). Primary VUR was the commonest anomaly followed by PUJO
and MCDK. The median ages of presentation of various anomalies are
summarized in Table I.
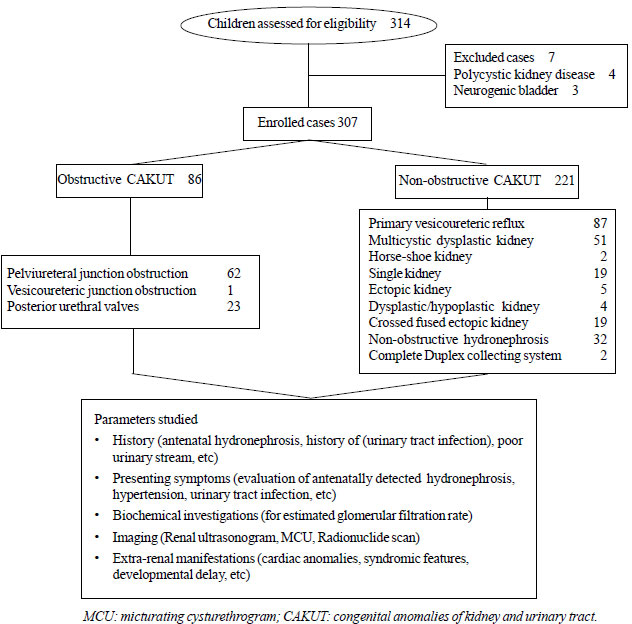 |
|
Fig. 1 Flow chart depicting
methodology of the study.
|
TABLE I Profile of Children with Congenital Anomalies of Kidney and Urinary Tract (N=307)
|
Diagnosis |
No. (%) |
Gender |
Age at diag- |
|
|
(male: |
nosis (mo) |
|
|
female) |
median (IQR) |
|
Primary VUR |
87 (28.3) |
54:33 |
10 (5, 27) |
|
Grade 1 |
4 |
|
|
|
Grade 2 |
35 |
|
|
|
Grade 3 |
31 |
|
|
|
Grade 4 |
14 |
|
|
|
Grade 5 |
3 |
|
|
|
PUJO
|
62 (20.1) |
42:20 |
7 (3, 22.5) |
|
Unilateral |
58 |
|
|
|
Bilateral |
4 |
|
|
|
MCDK |
51 (16.6) |
29:22 |
9 (2, 20) |
|
Right sided |
34 |
|
|
|
Left sided |
17 |
|
|
|
Non obstructive |
32 (10.4) |
22:10 |
8 (4, 12)
|
|
hydronephrosis* |
|
|
|
|
PUV |
23 (7.4) |
23:0 |
4 (2,14) |
|
Single kidney |
19 (6.1) |
13:6 |
13 (6, 36) |
|
Crossed fused
|
19 (6.1) |
11:8 |
10 (4, 36) |
|
ectopic kidney |
|
|
|
|
Left to right |
12 |
|
|
|
Right to left |
7 |
|
|
|
Ectopic kidney |
5 (1.6) |
2:3 |
12 (6,24) |
|
Dysplastic/Hypo- |
4 (1.3) |
4:0 |
30 (3.75, 70) |
|
plastic kidney |
|
|
|
|
Duplex collecting
|
2 (0.6) |
0:2 |
8 and 12# |
|
system |
|
|
|
|
Horse-shoe kidney |
2 (0.6) |
0:2 |
48 and 158# |
|
VUJO |
1 (0.3) |
1:0 |
25# |
|
PUV: Posterior urethral valve, PUJO: Pelviureteral junction
obstruction, MCDK: Multicystic dysplastic kidney, VUR:
Vesicoureteric reflux, VUJO: vesicoureteric junction
obstruction; *MCU and EC diuretic Renogram normal; #actual
ages. |
Out of the 307 children, 247 (80.4%) were detected
antenatally. Of this subgroup, 214 children had antenatal hydronephrosis.
The others included MCDK (n=26), single kidney (n=3),
crossed fused ectopic kidney (n=3) and ectopic kidney (n=1).
In 33 (10.7%) children, CAKUT was identified during work up for UTI,
while in 21 (6.8%), hypertension at presentation led to identification
of CAKUT. Eighteen children were incidentally detected to have CAKUT,
five were found to have CAKUT during evaluation for low eGFR, one child
during evaluation for poor urinary stream at 10 years of age, and one
child during evaluation for AKI. In two children, CAKUT was detected
during evaluation of nephrotic syndrome (one had primary VUR and another
had PUJO).
Among primary VUR (n=87), grade 3 was the
commonest. Primary VUR was bilateral in 29 (33.7%) children. Three
children (3.4%) were diagnosed during CKD work-up; 28 (32.1%) were
detected during workup for UTI. The mode of presentation of PUJO (n=62)
included antenatal detection in 90.4%; incidental detection in 6.5%,
following work up of UTI (1.6%) and during evaluation for nephrotic
syndrome (1.6%). Bilateral involvement was seen in 6%.Two children had
primary VUR in the contralateral kidney. Among MCDK cases (n=51),
8 children were incorrectly reported antenatally as hydronephrosis.
Three of these cases had primary VUR (2 in contralateral, 1 ipsilateral).
Among PUV cases (n=23), 86.9% had antenatal hydronephrosis; 78.2%
presented with low eGFR.
The median (IQR) ages of presentation for children
with PUV (n=23), VUR (n=87) and PUJO (n=62) were 4
(2,14) months, 10 (5,27) months and 7 (3, 22.5) months respectively.
Among these three CAKUT categories, 20 (86.9%), 54 (62.1%) and 56
(90.3%) had evidence of hydronephrosis in the antenatal ultrasounds.
Among the 307 enrolled children with CAKUT, weight
for age Z-score <-3 was noted in 29 (9.4%) children, while weight for
age Z-score between -2 to -3 was noted in 28 (9.1%) children.
Obstructive CAKUT presented earlier than
non-obstructive CAKUT (P=0.01) (Table II). A
greater proportion of obstructive CAKUTs were identified antenatally as
compared to non-obstructive CAKUTs (P=0.01). There was a male
preponderance in both groups. The proportions of children with UTI in
the obstructive versus non-obstructive CAKUT groups were 5.8% versus
12.6% respectively (P<0.01). The median eGFR for obstructive and
non-obstructive CAKUTs were 60 (48.3, 64.3) and 62.8 (60, 73.3) mL/min/1.73
m 2 (P<0.01).
TABLE II Comparison Between Obstructive and Non-obstructive Anomalies
|
Obstructive* |
Non-Obstructive |
P |
|
(n=86) |
**(n=221) |
value |
|
Antenatal diagnosis
|
77(89.5) |
170 (76.9) |
0.01 |
|
Male sex
|
56 (65.2) |
140 (63.3) |
0.02 |
|
UTI
|
5 (5.8) |
28 (12.6) |
<0.01 |
|
#$eGFR
|
60 (48.3,64.3) |
62.8 (60,73.3) |
<0.01 |
|
Hypertension at
|
7 (8.1) |
14 (6.3) |
0.12 |
|
presentation
|
|
|
|
|
#Age at diagnosis (mo)
|
7 (3,22.5) |
10 (4,24) |
0.01 |
|
*Comprised of Posterior urethral valve, Pelviureteral
junction obstruction, and Vesico-ureteric junction
obstruction;**comprised of primary vesicoureteric reflux,
Multicystic dysplastic kidney, Renal agenesis, Renal
hypo-dysplasia, pelvic kidney, horse shoe kidney, crossed fused
ectopia, Complete duplex collecting system; $in mL/min/173m2;
All values in n(%) except #median (IQR). |
Overall, 9 children (2.9%) had extrarenal
manifestations. One primary VUR case had Down syndrome, another had
VACTERL association. Three children with MCDK had cardiac anomalies
[ventricular septal defect (VSD): 2, atrial septal defect: 1], while 2
primary VUR cases had cardiac anomalies (VSD: 1, TOF:1). One child with
PUJO had VSD; and 1 child had developmental delay.
Discussion
This cross-sectional study assessed the clinical
profile in a cohort of 307 CAKUT cases. Obstructive CAKUTs presented
earlier causing significant impairment of e-GFR as compared to the
non-obstructive CAKUTs. This finding is comparable to earlier studies
[13,14]. Primary VUR was the commonest CAKUT followed by PUJO, MCDK,
non-obstructive hydronephrosis and PUV. In contrast, Soliman, et al.
[13], reported PUV (36.4%) as the commonest followed by primary VUR
(19.6%) and PUJO (18.7%). Aksu, et al. [15], reported PUJO in
62.7% followed by VUR in 16.6%. It is notable that we encountered quite
delayed presentations of various CAKUTs, particularly PUV and PUJO. This
can be inferred from the fact that though a majority of these CAKUTs had
evidence of antenatal hydronephrosis, their median age at presentation
to us was at a much later point of time. A significant number of
children had low eGFR or hypertension at presentation, which is
comparable to a previously published report [15]. Though a significant
number of children were detected antenatally, approximately 19.5% of the
CAKUTs had not been antenatally identified, and a majority of this
sub-group were identified after UTI.
We encountered significant number of MCDK cases
(16.6%) in contrast to previously reported studies [13], possibly due to
a referral bias. We also found only 2.9% prevalence of extrarenal
features in children with CAKUT. These extrarenal anomalies were
detected in primary VUR, PUJO and MCDK cases, but not in children with
PUV. This finding is different from a previous Egyptian report, wherein
57% of 107 CAKUT children had extrarenal features. The reasons for this
could be related to different ethnicity. The parental consanguinity in
our study cohort was 21% whereas it was 50.5% in this report [13].
There is paucity of published studies on the profile
of CAKUT from India; and this study provides valuable information
regarding this subject. Our study has few limitations. The study was
conducted at referral hospital and the profile of enrolled subjects may
not be representative of the disease profile at the community level.
Also, we could not perform genetic testing of patients enrolled in this
study.
It is known that a percentage of milder forms of
CAKUT can be diagnosed later in life. Nevertheless, the median ages of
presentation (despite a majority of them having had evidence of
antenatal hydronephrosis) suggests a delayed referral. This study
emphasizes the need for prompt referral by trained professionals in
order to initiate appropriate therapeutic strategies in children with
CAKUT and improvise the care bundle provided to these children.
Contributors: BHK: collected the data and
drafted the first version of the manuscript; SK: conceptualized the
study, was responsible for medical management of the cases, interpreted
the data and critically revised the manuscript. RA: confirmed the
radiological findings and critically reviewed the manuscript. VC:
responsible for surgical management of cases and critically reviewed the
manuscript. All authors approved the final version of the manuscript,
and are accountable for all aspects of the study. SK: shall act as
guarantor of the paper.
Funding: None; Competing interests: None
stated.
|
What This Study Adds?
•
The most common congenital anamalies of the kidney and
urinary tract (CAKUT) in our series were vesicoureteric reflux,
pelviureteric junction obstruction, multicystic dysplastic
kidney, non-obstructive hydronephrosis and posterior urethral
valves.
•
About one-fifth of anomalies had not been detected
antenatally.
•
Obstructive CAKUT presented earlier than non-obstructive
CAKUT.
|
References
1. Harambat J, van Stralen KJ, Kim JJ, Tizard EJ.
Epidemiology of chronic kidney disease in children. Pediatr Nephrol.
2012;27:363-73.
2. North American Pediatric Renal Transplant
Cooperative Study (NAPRTCS) (2008) 2008. Annual report. The EMMES
Corporation, Rockville, MD. Available from:
https://web.emmes.com/study/ped/annlrept/Annual%20 Report%20-2008.pdf.
Accessed May 14, 2019
3. Mong Hiep TT, Ismaili K, Collart F,
Damme-Lombaerts R, Godefroid N, Ghuysen MS, et al. Clinical
characteristics and outcomes of children with stage 3–5 chronic kidney
disease. Pediatr Nephrol. 2010;25:935-40.
4. Hattori S, Yosioka K, Honda M, Ito H, Japanese
Society for Pediatric nephrology. The 1998 report of the Japanese
National Registry data on pediatric end-stage renal disease patients. Pediatr
Nephrol. 2002;17:456-61.
5. KDIGO Clinical Practice Guidelines for Acute
Kidney Injury 2012. Available from: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.
pdf. Accessed May 14, 2019
6. Indian Society of Pediatric Nephrology,
Vijayakumar M, Kanitkar M, Nammalwar BR, Bagga A. Revised statement on
management of urinary tract infections. Indian Pediatr. 2011;48:709-17.
7. National Kidney Foundation. K/DOQI Clinical
Practice Guidelines for Chronic Kidney Disease: Evaluation,
Classification, and Stratification. Am J Kidney Dis. 2002;39:S1-266.
8. National High Blood Pressure Education Program
Working Group on High Blood Pressure in Children and Adolescents. The
Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood
Pressure in Children and Adolescents. Pediatrics. 2004;114:555-76.
9. Nguyen HT, Herndon CDA, Cooper C, Gatti J, Kirsch
A, Kokorowski P, et al. The Society for Fetal Urology Consensus
Statement on the Evaluation and Management of Antenatal Hydronephrosis.
J Pediatr Urol. 2010;6: 212-31.
10. Sinha A, Bagga A, Krishna A, Bajpai M, Srinivas
M, Uppal R, et al. Revised guidelines on management of antenatal
hydronephrosis. Indian Pediatr. 2013;50:215-31.
11. WHO child growth standards: Weight-for-age, World
Health Organization; 2006. Available from: https://
www.who.int/childgrowth/standards/weight_for_age/en/. Accessed May
14, 2019.
12. Lebowitz RL, Olbing H, Parkkulainen KV, Smellie
JM, Tamminen-Möbius TE. International System of Radiographic Grading of
Vesicoureteric Reflux. International Reflux Study in Children. Pediatr
Radiol. 1985;15:105-9.
13. Soliman NA, Ali RI, Ghobrial EE, Habib EI, Ziada
AM. Pattern of clinical presentation of congenital anomalies of the
kidney and urinary tract among infants and children. Nephrol (Carlton).
2015;20:413-8.
14. Ahmadzadeh A, Tahmasebi M, Gharibvand MM. Causes
and outcome of prenatally diagnosed hydronephrosis. Saudi J Kidney Dis
Transplant 2009;20:246-50.
15. Aksu N, Yavaþcan O, Kangin M, Kara OD, Aydin Y,
Erdoðan H, et al. Postnatal management of infants with
antenatally detected hydronephrosis. Pediatr Nephrol. 2005:20:1253.
|
|
|
 |
|

