|
|
|
Indian Pediatr 2018;55:573-575 |
 |
Hepatic and Cardiac
Iron-load in Children on Long-term Chelation with Deferiprone
for Thalassemia Major
|
|
Sidharth Totadri 1,
Deepak Bansal1,
Amita Trehan1,
Alka Khadwal2,
Anmol Bhatia3,
Kushaljit Singh Sodhi3,
Prateek Bhatia1,
Richa Jain1,
Reena Das4 and
Niranjan Khandelwal3
From 1Pediatric Hematology-Oncology Unit,
Department of Pediatrics, Advanced Pediatrics Center, 2Clinical
Hematology unit, Department of Internal Medicine and Departments of
3Radiodiagnosis and Imaging, and 4Laboratory
Hematology; Postgraduate Institute of Medical Education and Research,
Chandigarh, India.
Correspondence to: Dr Deepak Bansal, Professor,
Hematology-Oncology unit, Department of Pediatrics, Advanced Pediatrics
Center, Post Graduate Institute of Medical Education and Research,
Chandigarh, India.
Email: [email protected]
Received: November 13, 2017;
Initial review: March 16, 2018;
Accepted: April 28, 2018.
|
Objective: To evaluate the efficacy of prolonged deferiprone
monotherapy in patients with
b-thalassemia
major. Methods: This cross-sectional study included 40 patients
(age range 9 to 38 years) with thalassemia major receiving deferiprone
for ³5
years. Serum ferritin, and myocardial iron concentration (MIC) and liver
iron concentration (LIC) assessed by T2*MRI were recorded. Results:
The patients were receiving deferiprone for a mean (SD) duration of 12.1
(4.7) years. The median (IQR) dose of deferiprone was 85 (74.3, 95)
mg/kg/day. The MIC was normal or had a mild, moderate or severe
elevation in 29 (72.5%), 3 (7.5%), 3 (7.5%), and 5 (12.5%) patients. The
LIC was normal or had a mild, moderate or severe elevation in 2 (5%), 4
(10%), 11 (27.5%) and 23 (57.5%) patients. Conclusions: The
majority of patients receiving deferiprone had a moderate/severe hepatic
but normal cardiac iron load. Prolonged deferiprone monotherapy was
suboptimal for hepatic iron load in the majority.
Keywords: Cardiac failure, Hemosiderosis, Hepatic fibrosis,
Oral iron chelators.
|
|
I
n low- and middle-income countries (LMIC),
deferiprone is a popular and cost-effective option for iron chelation in
patients with b-thalassemia
major [1]. Desferrioxamine is seldom preferred due to its arduous route
of administration and cost [2]. Serum ferritin is commonly performed to
ascertain the iron overload in thalassemic patients. However, it is an
acute phase reactant, and a trend is more indicative than a single value
[3,4]. T2* magnetic resonance imaging (T2*MRI) has emerged as a
non-invasive tool, providing a more accurate estimate of cardiac and
hepatic hemosiderosis. We report the status of iron overload in patients
with thalassemia major who were receiving deferiprone monotherapy for a
prolonged duration.
Methods
This cross-sectional study was conducted in the
thalassemia day care center in Department of Pediatrics at PGIMER,
Chandigarh, India. Inclusion criteria were: (i) A diagnosis of
thalassemia major confirmed by hemoglobin electrophoresis or
high-performance liquid chromatography, (ii) receiving
deferiprone monotherapy for a duration of
³5 years, and (iii)
a T2*MRI performed in the previous 12 months. The exclusion criteria
included: (i) exposure to either desferrioxamine or deferasirox,
(ii) poor compliance (defined as >3 episodes of missing >25% of
the recommended dose/month) or a daily deferiprone dose <65 mg/kg. The
patients were enrolled between January 2017 and March 2017. The mean
serum ferritin over the previous 12 months was calculated. Serological
evidence for Human immunodeficiency virus (HIV), Hepatitis B (HBsAg) and
Hepatitic C (Anti-HCV-IgG) obtained in the preceding 12 months was also
recorded.
T2*MRI utilized a body coil on a 1.5-T magnetic
resonance scanner (MAGNETOM Aera, Siemens Healthcare). An elevation in
liver iron concentration (LIC) was categorized as: normal (<2 mg/g),
mild (2-7 mg/g), moderate (7-15 mg/g) or severe (>15 mg/g) [5]. An
elevation in myocardial iron concentration (MIC) was categorized as:
normal (<1.16 mg/g), mild (1.16-1.65 mg/g), moderate (1.65-2.71) or
severe (>2.71 mg/g) [5].
The study was approved by our Institute’s Ethics
Committee. An informed written consent was obtained from patients and/or
their caregivers.
Statistical analysis: The data was analyzed with
SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA). Comparison of
proportions was performed with either Chi-square or Fisher’s exact test.
The Spearman’s rank-order correlation coefficient was utilized for
assessment of correlation between MIC, LIC and serum ferritin.
Results
Forty patients were enrolled (Fig. 1).
The mean (SD) age was 23.4 (7.1) years (range 9-38 years). The mean (SD)
duration of receiving chelation with deferiprone was 12.1 (4.7) years
(range 5-22 years). Twenty-nine (72.5%) patients were receiving
deferiprone for ³10
years. The median (IQR) dose of deferiprone at enrolment was 85 (74.3,
95.0) mg/kg/day. The median (IQR) frequency of blood transfusions
administered to the patients was at 3 (3, 3) weekly interval. Thirteen
(32.5%) patients had undergone a splenectomy. Serology was reactive for
HBSAg, anti-HCV-IgG and HIV in 2 (5.3%), 4 (10.5%) and none of the 38
patients, respectively (reports were unavailable in two patients).
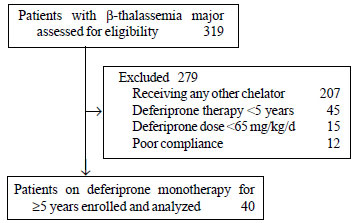 |
|
Fig. 1. Flow of patients in
the study.
|
The median (IQR) serum ferritin was 2271.5 (1369.0,
3089.3) ng/mL. The distribution of iron overload based on MIC and LIC is
illustrated in Table I. Amongst 32 patients with a
normal/mildly elevated MIC, 27 (84%) had a moderate/severe elevation in
LIC.
TABLE I Distribution of Cardiac and Liver Iron Overload in Pateients Receiving Deferiprone >5 years (N=40)
|
Iron
|
Myocardial iron
|
Liver iron
|
|
overload |
concentration (MIC) |
concentration(LIC) |
|
Normal |
29 (72.5) |
2 (5) |
|
Mild |
3 (7.5) |
4 (10) |
|
Moderate |
3 (7.5) |
11 (27.5) |
|
Severe |
5 (12.5) |
23 (57.5) |
|
Values in no(%). |
|
|
The serum ferritin correlated with LIC (r=0.499, P=0.001),
but not with MIC (r=0.280, P=0.080). Neither MIC nor LIC
correlated with the duration of deferiprone therapy.
Splenectomy did not influence cardiac (P=0.52)
or liver iron overload (P=0.29). Viral infections did not affect
cardiac (P=0.34) or liver iron overload (P=0.65). Presence
of moderate/severe cardiac or liver iron overload did not differ among
patients receiving £90
or >90 mg/kg/day of deferiprone (P=0.06 and 0.14, respectively).
Discussion
In this study, we report a group of patients
receiving deferiprone monotherapy – the mean duration of therapy
exceeding a decade. In our study, while serum ferritin demonstrated
correlation with LIC, there was a lack of correlation with MIC.
Three-fourths of the patients had a normal or a merely mildly increased
MIC. A moderate or severe iron overload was observed in 85% of the
patients.
The limitations of this study include a
cross-sectional design, and a lack of information on the dose of
deferiprone administered in the preceding years. A proportion of
patients were receiving deferiprone at a dose of 65-75 mg/kg/day.
Historical assessment of compliance can also be unreliable. Adverse
events of deferiprone were not described due to the cross-sectional
design of the study. There was a lack of comparison with patients
receiving desferrioxamine or deferasirox.
The cardiac chelating effect of deferiprone is
reiterated by our study. Studies evaluating its efficacy have
demonstrated improvement in myocardial hemosiderosis, comparable to or
superior to desferrioxamine [6,7]. International guidelines concur on
the importance of maintaining a low LIC in addition to MIC [3,8].
Several studies have demonstrated that deferiprone monotherapy lags
behind desferrioxamine and deferasirox in reducing LIC [9-12]. A
possible ‘bottleneck effect’, wherein, deferiprone is able to chelate
merely the iron available in the chelatable pool is postulated [13]. An
inferior iron chelation from the liver might in part be related to
inadequate doses of deferiprone [14]. However, in our study, even
patients receiving >90 mg/kg/day of deferiprone did not have a lower
liver iron overload.
We conclude that patients of thalassemia major
receiving long-term monotherapy with deferiprone have a high prevalence
of moderate or severe hepatic iron overload and a relatively low
prevalence of moderate or severe cardiac iron overload. Alternative
chelation strategies may be considered in patients with a high LIC on
monotherapy with deferiprone.
Contributors: ST: collected data, analyzed data
and drafted the manuscript; DB: conceptualized and designed the study,
collected data and edited the manuscript; AT, RJ: were pediatric
haematologists who contributed to clinical care and collection of
clinical data, supervised and edited the manuscript; AK: was the adult
haematologist who contributed to clinical care of older patients and
collection of clinical data, supervised and edited the manuscript; AB,
KSS: performed and reported T2*MRI scans, were responsible for
methodology of T2*MRI, supervised and edited the manuscript; NK:
standardized the software of the T2*MRI and reviewed all T2*MRI reports,
supervised and edited the manuscript; PB, RD: reported serum ferritin,
haemoglobin electrophoresis and high-performance liquid chromatography,
supervised and edited the manuscript.
Funding: None; Competing interest: None
stated.
|
What This Study Adds?
• Long term chelation with deferiprone
monotherapy in patients with
b-thalassemia
major was found to be suboptimal for liver iron overload in
majority of the patients.
|
References
1. Verma IC, Saxena R, Kohli S. Past, present and
future scenario of thalassaemic care & control in India. Indian J Med
Res. 2011;134:507-21.
2. Kwiatkowski JL. Real-world use of iron chelators.
Hematol Am Soc Hematol Educ Program. 2011;2011: 451-8.
3. Cappellini MD, Cohen A, Porter J, Taher A,
Viprakasit V. Guidelines for the Management of Transfusion Dependent
Thalassemia. 3rd ed. Nicosia, Cyprus: Thalassaemia International
Federation; 2014.
4. Kolnagou A, Yazman D, Economides C, Eracleous E,
Kontoghiorghes GJ. Uses and limitations of serum ferritin, magnetic
resonance imaging T2 and T2* in the diagnosis of iron overload and in
the ferrikinetics of normalization of the iron stores in thalassemia
using the International Committee on Chelation deferiprone/deferoxamine
combination protocol. Hemoglobin. 2009;33:312-22.
5. Mandal S, Sodhi KS, Bansal D, Sinha A, Bhatia A,
Trehan A, et al. MRI for quantification of liver and cardiac iron
in thalassemia major patients: Pilot study in Indian population. Indian
J Pediatr. 2017;84:276-82.
6. Pennell DJ, Berdoukas V, Karagiorga M, Ladis V,
Piga A, Aessopos A, et al. Randomized controlled trial of
deferiprone or deferoxamine in beta-thalassemia major patients with
asymptomatic myocardial siderosis. Blood. 2006;107:3738-44.
7. Kuo KH, Mrkobrada M. A systematic review and
meta-analysis of deferiprone monotherapy and in combination with
deferoxamine for reduction of iron overload in chronically transfused
patients with b-thalassemia.
Hemoglobin. 2014;38:409-21.
8. Saliba AN, Harb AR, Taher AT. Iron chelation
therapy in transfusion-dependent thalassemia patients: current
strategies and future directions. J Blood Med. 2015;6:197-209.
9. Pepe A, Meloni A, Rossi G, Cuccia L, D’Ascola GD,
Santodirocco M, et al. Cardiac and hepatic iron and ejection
fraction in thalassemia major: Multicentre prospective comparison of
combined deferiprone and deferoxamine therapy against deferiprone or
deferoxamine monotherapy. J Cardiovasc Magn Reson. 2013;15:1.
10. Perifanis V, Christoforidis A, Vlachaki E, Tsatra
I, Spanos G, Athanassiou-Metaxa M. Comparison of effects of different
long-term iron-chelation regimens on myocardial and hepatic iron
concentrations assessed with T2* magnetic resonance imaging in patients
with beta-thalassemia major. Int J Hematol. 2007;86:385-9.
11. Berdoukas V, Chouliaras G, Moraitis P, Zannikos
K, Berdoussi E, Ladis V. The efficacy of iron chelator regimes in
reducing cardiac and hepatic iron in patients with thalassaemia major: a
clinical observational study. J Cardiovasc Magn Reson. 2009;28;11:20.
12. Pepe A, Meloni A, Capra M, Cianciulli P,
Prossomariti L, Malaventura C, et al. Deferasirox, deferiprone
and desferrioxamine treatment in thalassemia major patients: cardiac
iron and function comparison determined by quantitative magnetic
resonance imaging. Haematologica. 2011;96:41-7.
13. Fischer R, Longo F, Nielsen P, Engelhardt R,
Hider RC, Piga A. Monitoring long-term efficacy of iron chelation
therapy by deferiprone and desferrioxamine in patients with beta-thalassaemia
major: application of SQUID biomagnetic liver susceptometry. Br J
Haematol. 2003;121:938-48.
14. Kwiatkowski JL, Belmont A. Deferiprone for the
treatment of transfusional iron overload in thalassemia. Expert Rev
Hematol. 2017;10:493-503.
|
|
|
 |
|

