|
|
|
Indian Pediatr 2017;54: 556-559 |
 |
Candida Blood Stream
Infection in Neonates: Experience from A Tertiary Care Teaching
Hospital of Central India
|
|
Sriparna Basu, Rajesh Kumar, *Ragini Tilak and Ashok
Kumar
From Departments of Pediatrics and *Microbiology,
Institute of Medical Sciences, Banaras Hindu University, Varanasi,
India.
Correspondence to: Dr. Sriparna Basu, Professor,
Neonatal Unit, Department of Pediatrics, Institute of Medical Sciences,
Banaras Hindu University, Varanasi 221 005, India.
Email:
drsriparnabasu@rediffmail.com
Received: June 15, 2016;
Initial review: October 14, 2016;
Accepted: February 23, 2017.
Published online:
March 29, 2017.
PII:S097475591600061
|
Objective: To assess the epidemiology of neonatal
Candida blood stream infection. Methods: Medical records of
neonates with Candida blood stream infection over 5 years
(September 2010 to August 2015) were reviewed. Clinical details, species
distribution and antifungal susceptibility were noted. Results:
114 neonates developed Candida blood stream infection. Commonly isolated
Candida species were C. tropicalis, C. albicans and C.
parapsilosis. Susceptibility for fluconazole and amphotericin B was
86.6% and 68.3%, respectively. Central line >7 days and hospital stay
>28 days were independent risk factors associated with non-albicans
Candida infection. Conclusions: Early removal of central
line, timely fungal culture and antifungal susceptibility are necessary
for early and appropriate treatment and better outcome.
Key words: Blood stream infection, Neonate, Outcome,
Risk factors.
|
|
C
andida blood stream infection (BSI) is an
important cause of neonatal sepsis and sepsis-related mortality [1].
Common risk factors for Candida BSI include prematurity and very low
birth weight (VLBW), central vascular catheterization, parenteral
nutrition, use of broad-spectrum antibiotics, H2
blockers and corticosteroids, endotracheal intubation, and prolonged
hospital stay [1,2]. Although C. albicans accounts for 45–55% of
Candida BSI among infants [1,3,4], recent studies have detected a shift
towards non-albicans Candida (NAC) species [3-5], which are often
associated with high mortality and poor antifungal susceptibility [5-7].
To evaluate the disease burden and plan for an early and effective
intervention, a thorough knowledge of the local epidemiology of Candida
infection is critical. The present study was undertaken to assess the
species distribution, susceptibility pattern, risk factors and outcome
of neonates developing Candida BSI during hospital stay.
Methods
Medical records of all inborn neonates who
developed Candida BSI over a period of 5 years (September 2010 to August
2015) were reviewed. Candida BSI was defined as at least one pure growth
of Candida species in blood culture [8] within 72 hours of
inoculation, in presence of clinical features suggestive of sepsis such
as respiratory distress/apnea, tachycardia/bradycardia, poor perfusion,
feeding intolerance, temperature instability, lethargy, or seizures [9].
Culture positivity within 14 days, with the same Candida species
was considered to be the same infection episode [2]. The study protocol
was approved by the Institute Ethics Committee.
Clinical and investigation details, treatments
received, response to therapy and outcome were noted. For blood culture,
paired samples were inoculated in sheep brain heart infusion broth (Himedia,
Mumbai, India) in 1:10 dilution and incubated at 37°C for 48 h. Any
growth observed was subcultured on 5% sheep blood agar, MacConkey’s
agar, and Sabouraud’s dextrose agar (SDA) with chloramphenicol (0.05%).
Species was identified by colony morphology on SDA, color production on
chromogenic media, growth at 45°C, germ tube test, chlamydospore
formation, and carbohydrate fermentation and assimilation tests.
Antifungal susceptibility was determined by the Clinical Laboratory
Standards Institute disk diffusion testing [10]. Statistical analysis
was done using SPSS 16. Risk factors were analyzed by univariate and
stepwise multivariate logistic regression analysis.
Results
During the period of review, 13346 neonates were
delivered and 3128 were admitted in the Neonatal Unit. Candida species
was isolated in blood culture of 114 neonates (0.9% of total deliveries
and 3.6% of total admissions); 4.9% of VLBW and 11.2% of extremely-low
birth weight neonates developed candidemia. Speciation could be done in
82 isolates, C. Tropicalis, 32 (39%), C. albicans, 29
(35.4%), C. parapsilosis, 10 (12.2%), C. glabrata, 5
(6.1%), C. krusei, 4 (4.8%) and C. guilliermondii, 2
(2.4%).
NAC BSI was associated with lower mean gestational
age. Common presentations of Candida BSI were lethargy, bleeding
manifestations, intraventricular hemorrhage, feeding intolerance and
pneumonia or apnea. Overall, the combined incidence of multi-system
involvement including meningitis, renal candidiasis/urinary tract
infection, septic arthritis, endocarditis, endophthalmitis and
Fournier’s gangrene was higher with NAC than C. albicans, though
no difference was observed in the incidence of individual morbidity.
Mortality and mean duration of hospital stay were significantly higher
in NAC (Table I). Positive sepsis screen was documented in
only 18 (15.8%), but the incidence of raised C-reactive protein (>10
mg/L) and severe thrombocytopenia (<50000/µL) was high, 92 (80.7%) and
68 (59.6%), respectively.
TABLE I Details of Neonates With Candida Bloodstream Inection
|
Parameter |
All neonates with |
Neonates with |
Neonates with
|
P value*
|
|
Candida BSI
|
Candida albicans |
Candida non-
|
|
|
(n = 114) |
BSI (n = 29) |
albicans BSI |
|
|
|
|
(n = 53) |
|
|
Chorioamnionitis, n (%) |
17 (14.9) |
5 (17.2) |
9 (17.0) |
1.0 |
|
Mode of delivery
|
|
SVD, n (%) |
71 (62.3) |
18 (62.1) |
33 (62.3) |
1.0 |
|
Cesarean section, n (%) |
43 (37.7) |
11 (37.9) |
20 (37.7) |
|
|
Birth weight (g), mean (SD)
|
1235 (485) |
1280 (520) |
1125 (482) |
0.18 |
|
Gestational age (wk), mean (SD) |
30.6 (1.4) |
30.8 (2.1) |
29.7 (2.0) |
0.02 |
|
Male: Female |
1:1 |
1.1:1 |
1:1 |
0.90 |
|
Apgar score |
|
1 min, median (IQR) |
6 (5-7) |
6 (5-7) |
6 (5-7) |
1.0 |
|
5 min, median (IQR) |
8 (7 - 9) |
8 (7 - 9) |
8 (7 - 9) |
|
|
Late-onset sepsis, n (%) |
94 (82.5)
|
23 (79.3) |
52 (98.1) |
0.007 |
|
Clinical presentations |
|
Lethargy, n (%) |
41 (36.0) |
16 (55.2) |
22 (41.5) |
0.26 |
|
Bleeding manifestations, n (%) |
39 (34.2) |
10 (34.5) |
24 (45.3) |
0.36 |
|
IVH, Grade I-II, n (%) |
38 (33.3) |
12 (41.4) |
21(41.5) |
0.49 |
|
IVH, Grade III-IV, n (%) |
10 (5.3) |
2 (6.9) |
6 (11.3) |
0.71 |
|
Feed intolerance, n (%) |
38 (33.3) |
10 (34.5) |
17 (32.1) |
1.0 |
|
Pneumonia/apnea, n (%) |
34 (29.8) |
8 (27.6) |
21(39.6) |
0.34 |
|
Multi-system involvement, n (%) |
40 (35.1) |
6 (20.7) |
27 (50.9) |
0.009 |
|
Duration of hospital stay (d), mean (SD) |
24.8 (10.4) |
18.7 (14.2) |
32.4 (15.3) |
<0.001 |
|
Death, n (%) |
17 (14.9) |
2 (6.9) |
14 (26.4) |
0.041 |
|
Follow up |
|
PVL, n (%) |
13 (11.4) |
4 (13.8) |
8 (15.1) |
0.10 |
|
ROP, n (%) |
11 (9.6) |
3 (10.3) |
7 (13.2) |
0.10 |
|
Abnormal BERA, n (%) |
2 (1.8) |
0 (0.0) |
2 (3.8) |
0.54 |
|
Abnormal DDST, n (%) |
8 (7.0) |
1 (3.4) |
6 (11.3) |
0.41 |
|
*Comparison was made between neonates with Candida albicans BSI and neonates with Candida non-albicans BSI, BSI – Blood stream infection, SVD – Spontaneous vaginal delivery, SD – Standard deviation, IQR – Inter Quartile Range, PVL - Periventricular leucomalacia, ROP - Retinopathy of prematurity, BERA - brainstem evoked response audiometry, DDST - Denever Developmental Screening test; IVH: Intraventricular hemorrhage. |
On univariate analysis, risk factors significantly
associated with NAC BSI were nil orally >5 days (OR 0.224, 95% CI 0.059,
0.842], mechanical ventilation >5 days (OR 0.187, 95% CI 0.039, 0.889),
central line >7 days (OR 0.166, 95% CI 0.050, 0.543), intralipid
infusion >7 days (OR 0.225, 95% CI 0.068, 0.740), and hospital stay >28
days (OR 0.217, 95% CI 0.079, 0.591). On step-wise logistic regression
analysis, central line >7 days and hospital stay >28 days were
independent predictors of NAC BSI (Table II).
TABLE II Step-wise Multiple Logistic Regression Analysis of Risk Factors for Candida
Non-albicans (NAC) Blood Stream Infections
|
Risk factor |
Adjusted Odds Ratio (95% CI) |
|
Nil orally >5 d |
1.0 (0.18-5.30) |
|
Mechanical ventilation >5 d |
4.4 (0.81-24.75) |
|
Central line >7 d |
4.3 (1.07-17.32) |
|
20% intralipid infusion >7 d |
1.8 (0.47-4.43) |
|
Use of >2 broad spectrum antibiotics |
1.1 (0.32-3.80) |
|
Prolonged stay in hospital (>28 d) |
3.1 (1.05-9.74) |
|
*The model was statistically significant, and explained
32.6% (Nagelkerke R2) of the total variance in NAC BSI and
correctly classified 76.8% of cases. |
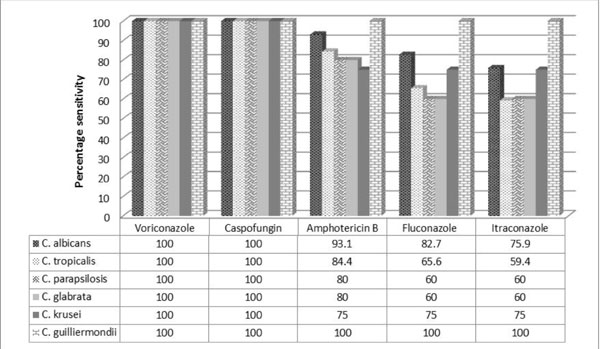
Intravenous fluconazole was the first empirical
antifungal used on clinical suspicion of fungal sepsis and liposomal
amphotericin B was the second line. Change of antifungal chemotherapy
was based on clinical deterioration or susceptibility testing. No
antifungal chemoprophylaxis was given. Voriconazole and caspofungin
demonstrated 100% susceptibility, whereas overall sensitivity for
amphotericin B, fluconazole and itraconazole were 86.6%, 68.3% and
67.1%, respectively. C. parapsilosis and C. tropicalis
demonstrated least susceptibility to fluconazole and amphotericin B (Fig.
1). 41.2% of Candida isolates from the neonates who expired were
resistant to both fluconazole and amphotericin-B.
 |
|
Fig. 1
Percentage sensitivity of Candida spp. to antifungal drugs.
|
Discussion
In the present study the incidence of Candida BSI was
0.9% of total deliveries and 3.6% of total admissions. C. tropicalis
was the most commonly isolated Candida species, followed by C.
albicans and C. parapsilosis. The incidence of mortality and
duration of hospital stay were significantly higher in NAC BSI. Central
line >7 days and hospital stay >28 days were independent predictors of
NAC BSI. C. parapsilosis and C. tropicalis demonstrated
higher resistance to fluconazole and amphotericin-B.
The major limitations of our study are its
retrospective design, and failure to perform species identification in
all cases. We observed a wide spectrum of multi-system involvement,
long-term complications and species distribution. Emergence of NAC as a
common cause of candidemia has been reported by previous Indian studies
[8,11-13]. C. parapsilosis was identified as the most common
fungal species in neonates in earlier reports, which is in contrast to
our observation [8]. C. tropicalis is virulent and is the second
leading cause of candidemia in adults, but is quite infrequent among
neonates [14]. Overall, resistance to fluconazole and amphotericin B was
similar to previous studies [8]. Compared to other Indian studies [8],
mortality was less in our study, but high incidence of resistance to
both fluconazole and amphotericin B amongst infants who died was noted.
To conclude, emergence of NAC species and their
association with higher mortality and longer duration of hospital stay
is a cause for concern. Higher resistance of C. tropicalis and
C. parapsilosis to fluconazole and amphotericin B is alarming.
Prevention of risk factors in susceptible neonates with early removal of
central line, timely fungal culture, Candida speciation and
susceptibility testing are necessary for appropriate institution of
treatment and better outcome. Frequent empirical use of fluconazole and
amphotericin B may be avoided as it may lead to a shift in species
distribution and higher antifungal resistance.
Contributors: SB: was involved in study planning,
design and writing of the manuscript. RK: was involved with the data
collection, analysis and writing of the manuscript. RT: was involved
with the microbiological analysis and writing of the manuscript. AK: was
involved with the study planning and writing of the manuscript. All
authors approved the final version of the manuscript. SB will act as the
guarantor of the paper.
Funding: None; Competing interest: None
stated
|
What This Study Adds?
• Non-albicans Candida infection was
associated with higher mortality and increased duration of
hospital stay.
• Both C. parapsilosis and C.
tropicalis demonstrated higher resistance to fluconazole and
amphotericin B.
|
References
1. Benjamin DK Jr, Stoll BJ, Gantz MG, Walsh MC, Sánchez
PJ, Das A, et al; Eunice Kennedy Shriver National Institute of
Child Health and Human Development Neonatal Research Network. Neonatal
candidiasis: epidemiology, risk factors, and clinical judgment.
Pediatrics. 2010;126:e865-73.
2. Oeser C, Vergnano S, Naidoo R, Anthony M, Chang
J, Chow P, et al; Neonatal Infection Surveillance Network (neonIN).
Neonatal invasive fungal infection in England 2004-2010. Clin Microbiol
Infect. 2014;20:936-41.
3. Chitnis AS, Magill SS, Edwards JR, Chiller TM,
Fridkin SK, Lessa FC. Trends in Candida central line-associated
bloodstream infections among NICUs, 1999–2009. Pediatrics.
2012;130:e46-52.
4. Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan
T. Changing incidence of Candida bloodstream infections among NICU
patients in the United States: 1995-2004. Pediatrics. 2006;117:1680-7.
5. Steinbach WJ, Roilides E, Berman D, Hoffman JA,
Groll AH, Bin-Hussain I, et al. Results from a prospective,
international, epidemiologic study of invasive candidiasis in children
and neonates. Pediatr Infect Dis J. 2012; 31:1252-7.
6. Makhoul IR, Kassis I, Smolkin T, Tamir A, Sujov P.
Review of 49 neonates with acquired fungal sepsis: further
characterization. Pediatrics. 2001;107:61-6.
7. Pammi M, Holland L, Butler G, Gacser A, Bliss JM.
Candida parapsilosis is a significant neonatal pathogen: a systematic
review and meta-analysis. Pediatr Infect Dis J. 2013; 32:e206-16.
8. Juyal D, Sharma M, Pal S, Rathaur VK, Sharma N.
Emergence of non-albicans Candida species in neonatal candidemia. N Am J
Med Sci. 2013;5:541-5.
9. Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman
L, Polin RA. Time for a neonatal-specific consensus definition for
sepsis. Pediatr Crit Care Med. 2014;15:523-8
10. National Committee for Clinical Laboratory
Standards. Methods for antifungal disk diffusion susceptibility testing
yeast: Approved guideline M-44A. Wayne, PA: NCCLS; 2004.
11. Xess I, Jain N, Hasan F, Mandal P, Banerjee U.
Epidemiology of candidemia in a tertiary care centre of North India:
5-year study. Infection. 2007;35:256-9.
12. Kothari A, Sagar V. Epidemiology of Candida
bloodstream infections in a tertiary care institute in India. Indian J
Med Microbiol. 2009;27:171-2.
13. Sardana V, Pandey A, Madan M, Goel SP, Asthana
AK. Neonatal candidemia: A changing trend. Indian J Pathol Microbiol.
2012;55:132-3.
14. Roilides E, Farmaki E, Evdoridou J, Francesconi
A, Kasai M, Filioti J, et al. Candida tropicalis in a
neonatal intensive care unit: Epidemiologic and molecular analysis of an
outbreak of infection with an uncommon neonatal pathogen. J Clin
Microbiol. 2003;41:735-41.
|
|
|
 |
|

