|
|
|
Indian Pediatr 2016;53: 601-606 |
 |
Rotavirus-specific
Salivary and Fecal IgA in Indian Children and Adults
|
|
Anu Paul, Sudhir Babji, Rajiv Sarkar, Robin Penugula
Lazarus and Gagandeep Kang
From The Wellcome Trust Research Laboratory, Division
of Gastrointestinal Sciences, Christian Medical College,
Vellore TN, India.
Correspondence to: Dr Gagandeep Kang, The Wellcome
Trust Research Laboratory, Division of Gastrointestinal Sciences,
Christian Medical College, Vellore Tamil Nadu 632 004, India.
Email: [email protected]
Received: July 09, 2015;
Initial review: August 22, 2015;
Accepted: May 07, 2016.
|
Objective: To compare serum, salivary and fecal IgA responses in
infants and adults following rotavirus vaccination.
Study design: Laboratory testing of samples from
clinical trials.
Setting: Medical College Hospital.
Participants: 13 healthy adult volunteers not
given vaccine, 20 healthy adult volunteers given one dose of bovine
rotavirus tetravalent vaccine (Shantha Biotechnics), and 88 infants
given 3 or 5 doses of Rotarix.
Outcome measures: Serum, salivary and fecal IgA
at one or more time points.
Methods: IgA antibodies were estimated in serum,
saliva and fecal samples by enzyme-linked immunosorbent assay, and
normalized to total IgA in saliva.
Results: In naturally infected adult volunteers,
comparing serum and salivary IgA showed significant positive correlation
(r=0.759; P=0.003). Of 20 vaccinated adults, complete samples
showing change were available for 10; among them there was a significant
positive correlation (P<0.05) between pre-vaccination serum and
pre-vaccination salivary IgA but not between post-vaccination serum and
post-vaccination salivary IgA. Of 88 infants given 3 or 5 doses of
vaccine, 13 had more than 4-fold IgA response in serum, saliva and fecal
samples, 6 had a 2-4 fold increases in all specimens. There was weak
correlation between seroconversion rates measured by serum and salivary
antibody responses. Salivary and stool assays were able to detect
seroconversion in a few children in whom there was no detectable
response in serum.
Conclusions: Evaluation of multiple samples is
useful for intensive experimental study designs and may help improve our
understanding of the induction and dynamics of immune responses to
rotavirus vaccination.
Keywords: Antibody, Immunity, Serum, Vaccine.
|
|
All live attenuated rotavirus vaccines have been
developed based on evidence that natural rotavirus infections elicit
protective immune responses, particularly against severe rotavirus
disease [1]. There are two currently available oral rotavirus vaccines
licensed in over 100 countries [2-4], as well as nationally licensed
vaccines in India, Vietnam and China. For a period after rotavirus
vaccination, much of the serum IgA is expected to be rotavirus-specific
as in natural rotavirus infections [5]. Although there is no defined
correlate of protection, serum IgA is the most widely available measure
of seroconversion so far, and has been used to measure immunogenicity of
all candidate rotavirus vaccines [6,7]. As rotavirus is a mucosal
pathogen infecting the epithelial cells of small intestinal villi,
mucosal gut antibodies could be expected to be reliable indicators of
immune response following natural rotavirus infection or rotavirus
vaccination [8].
Measuring local immunity in the small intestine is
considered the most sensitive marker of rotavirus infection; although
obtaining duodenal fluid from children by intubation is inappropriate,
and therefore other surrogate markers that accurately reflect intestinal
immune responses are necessary. Detecting rotavirus-specific antibodies
in feces, saliva and other suitable body fluids has been proposed as a
non-invasive alternative [5,8-11]. Secretory IgA responses in secretions
from sublingual glands (non-parotid glands) may better reflect B cell
induction in gut-associated lymphoid tissue (GALT) than the parotid
response, which may be more strongly linked to immune induction in
nasal-associated lymphoid tissue (NALT) [12]. Saliva collection is easy,
rapid, requires less training and eliminates the need for blood draws.
Further, previous studies suggest that in settings with low vaccine
immunogenicity, measuring salivary antibody responses to vaccination
might add to the apparent vaccine ‘take’ rate determined by detection of
serum antibodies alone [13,14].
Methods
Rotavirus-specific serum and salivary IgA antibody
levels were measured in healthy adult volunteers and infants and adults
vaccinated with either Rotarix or Bovine-Human Reassortant Rotavirus
Vaccine (BRV-TV). Fecal IgA was measured in infants given Rotarix in
previously published studies [15,16] conducted at our institute after
approval by the Institutional Review Board and with written informed
consent from participants or from their parents.
Healthy adults, presumed to be previously exposed who
did not receive rotavirus vaccine: A pilot study with collection of
serum and saliva was carried out in 13 healthy adult (6 female)
volunteers in order to assess the use of a Phosphate buffered saline
(PBS)-based antibody transport medium to extract and process saliva
which could be stored at -20°C and later be used to measure rotavirus
-specific IgA antibody levels. One saliva sample was collected from each
healthy adult volunteer using Oracol swabs (Malvern Medical Developments
Ltd, Worcester, UK). The swab was placed between the lower cheek and
gums of the volunteers and gently rubbed back and forth for about 1
minute until the absorbent sponge was moist. Salivary and serum IgA
antibody levels were compared.
Healthy adult volunteers who received one dose of
rotavirus vaccine: Rotavirus-specific serum and salivary IgA
antibody responses before and after vaccination with a BRV-TV were
measured in 20 healthy Indian adults who were given a single dose of
BRV-TV as previously reported [16]. From the 20 adult volunteers in the
Phase 1 BRV-TV study, parotid and sublingual salivary secretions and
whole saliva were collected prior to vaccination and the second sample
set 28-30 days post-vaccination. Prior to collection, all volunteers
filled a questionnaire, which documented time of last beverage intake
and absence of dental/gum bleeding. They then rinsed their mouths
thoroughly with water. Two sorbettes (Salimetrics Item No. 5029.00) were
placed together under the tongue or between gums and cheek for 60
seconds in each site, to obtain the secretions from sub lingual and
parotid glands, respectively; the sorbettes were stored as described by
the manufacturer. Volunteers were also instructed to pool saliva in
their mouths for about 90 seconds before collecting the whole saliva
into a sterile storage tube. The storage tubes were centrifuged for 15
minutes at 3000-3500 rpm to extract the saliva. The processed saliva was
recovered; protease inhibitors were added and mixed thoroughly. All
processed oral fluid specimens were stored at -20°C until testing.
Healthy infants who received 3 or 5 doses of
rotavirus vaccine: Comparison of rotavirus specific serum, salivary
and fecal IgA antibody responses before and after vaccination with 3 or
5 doses of Rotarix was studied in 90 infants recruited at 6 weeks of age
and enrolled in a Phase 4 randomized, parallel group comparison study as
described previously [15]. Two saliva samples were collected from all
vaccinated infants, one prior to administration of the first dose of
Rotarix and the second was collected 28 days after the last (3rd or 5th)
dose of vaccine administration. Saliva was collected using Salimetric
Infant swabs (Item no: 5001.08) and storage tubes (Item no: 5001.05).
Specimens were transported to the laboratory in ice packs. A total of 88
infants (44 in each arm) had a complete set of serum and saliva samples
available. Salivary IgA levels were also compared with fecal IgA levels
in a subset of vaccinated infants, from whom stool samples were
collected to study vaccine shedding.
Sample collection and Processing
Blood: Approximately 5 mL and 3.5 mL of venous
blood were obtained by trained phlebotomists prior to and 28-30 days
after oral rotavirus vaccine immunization from adult volunteers and
infants, respectively. Serum was separated and stored at -20 ºC till
testing.
Saliva: To each tube containing either an Oracol
or a Salimetrics swab, 1 mL of antibody transport medium (containing
0.2% Tween 20, 10% fetal bovine serum (FBS) and 0.7% Antibiotic/antimycotic,
in phosphate buffered saline, pH 7.2) was added, vortexed for 20 seconds
and centrifuged at 3000 rpm for about 10 minutes. After centrifugation,
the processed saliva was recovered and the oral fluid was stored at
-20°C until testing.
Stool: Following the first immunization,
mothers/guardians of immunized infants were requested to collect
approximately 5 g of stool, at 0, 3 and 7 days after each dose of
immunization, except the first where there was no 0 day sample. These
stool samples were stored at -70°C until tested. Fecal IgA was measured
in a subset of stool specimens obtained from infants who were either
baseline sero-negative and responded to vaccination (n=12), or
infants who were baseline sero-positive but had a >4 fold increase in
serum IgA from baseline (n=11).
Measuring Rotavirus-specific IgA
All processed serum, salivary and fecal samples were
tested in an antibody-sandwich, enzyme linked immunosorbent assay
(ELISA) using the bovine G6P [5] WC3 strain or the human G1P[8]
rotavirus antigen. The procedure for measurement of rotavirus specific
IgA expressed as was used [16,17], and specimen processing modified for
saliva and fecal specimens. Undiluted neat processed saliva in antibody
transport medium (supernatants and stool samples diluted in 1% bovine
serum albumin (supernatants from 10% homogenized suspensions centrifuged
at 3000 rpm for 15 min) were assayed to measure rotavirus-specific
salivary and fecal IgA respectively. Total salivary IgA was also
measured in all saliva samples so that the amount of rotavirus-specific
IgA could be normalized to 1 mg of total IgA present in the processed
specimen. The results for the rotavirus-specific salivary IgA
measurement were expressed as rotavirus IgA U/mL saliva in antibody
transport medium. The final concentrations of stool rotavirus IgA was
calculated by normalizing the total IgA concentration present in one
milligram of stool.
Among vaccinated infants, seroconversion or a
positive response was considered as the development of detectable (i.e.
³20 U/mL)
rotavirus-specific serum anti-rotavirus IgA antibodies in the serum 28
days after immunization, from a baseline of negative (<20 U/mL) or no
detectable antibody levels. Sero-response, as a measure of response to
the vaccine not meeting criteria for seroconversion, was indicated as a
two- or greater-fold increase in antibody titres in serum or body fluids
as compared to baseline.
Measuring Total IgA
To measure total salivary IgA concentrations, wells
of microtiter plates were coated overnight with either rabbit anti-human
IgA (Sigma, St Louis, MO) or normal rabbit serum (DakoCytomation,
Glostrup, Denmark) as negative control. The next day known
concentrations of serially diluted purified human IgA (Sigma, St Louis,
MO.) standards and doubling dilutions of salivary secretions diluted in
1% skimmed milk or supernatants from the 10% stool suspensions was added
and incubated for an hour. This was followed by washing and the addition
of biotinylated rabbit anti-human IgA. After incubating for an hour,
plates were washed and peroxidase-conjugated avidin-biotin was added and
incubated. The substrate (orthophenylenediamine/ H 2O2)
was added after washing plates and incubated in dark. After 30 min, the
reaction was stopped with 1M H2SO4
and absorbance OD at 492 nm was measured. Total salivary IgA
concentrations were determined from the plotted values for standard IgA
concentrations. The final concentrations of salivary or fecal rotavirus
IgA were calculated and expressed by normalizing to the total IgA
concentration present in one milliliter of saliva in antibody transport
medium or relative rotavirus IgA unit per milligram of total IgA [17],
respectively.
Statistical analysis: Statistical analyses were
done using GraphPad Prism, Version 4.0. Fold changes were calculated as
a ratio between the post- and the pre-immunization sample. The
rotavirus-specific serum, salivary and fecal IgA units derived from the
optical density measurements were compared. Pearson’s correlation
coefficients were computed after log transformation and assessed for
significance. Where IgA was undetectable, a constant number (1) was
added to all the IgA values to make them non-zero values for calculation
of geometric mean concentrations (GMC) and prior to log transformations,
if required. P<0.05 was considered statistically significant.
Results
Eleven of the 13 adult volunteers were positive for
serum rotavirus IgA while 10 had detectable levels of rotavirus specific
salivary IgA. The GMC of rotavirus specific serum IgA was 98.9 whereas
that of salivary IgA was 1.4. There was a significant positive
correlation (r=0.759; P=0.003) between the serum and salivary IgA
units.
From 20 volunteers who received BRV-TV
vaccine/placebo, 89 salivary secretions were tested for rotavirus-
specific IgA and compared with the corresponding serum rotavirus IgA
levels (Table I), with all samples available for only 10
volunteers. Of the 10 sets of pre-post parotid secretions that were
tested, >2-fold response was seen in 5/10 sublingual and 4/10 whole
saliva samples, but only 2/10 parotid responses. There was a significant
positive correlation (P<0.05) between pre-vaccination serum and
pre-vaccination saliva, but not between post-vaccination serum and
post-vaccination salivary IgA. Pre- and post-vaccination rotavirus
specific IgA was significantly correlated (r= 0.9, P<0.01)
between parotid and non-parotid secretions. For whole saliva, the
post-vaccination samples alone had a positive correlation of IgA levels
in parotid and non-parotid secretions (r=0.8, P <0.05).
TABLE I Rotavirus-specific Salivary IgA in Parotid, Sub-lingual and Whole Saliva Secretions in
Adult Volunteers (N=20) given a Single Dose of Bovine Rotavirus Tetravalent Vaccine
|
Pre-Post vaccination |
Number of volunteers showing rota- |
|
salivary IgA response |
virus -specific salivary IgA response
|
|
Parotid |
Sub-lingual |
Whole |
|
secretions |
secretion |
saliva |
|
<2 - fold |
8 |
5 |
15 |
|
2- 4 fold |
1 |
4 |
3 |
|
>4 - fold |
1 |
1 |
1 |
|
NA |
10 |
10 |
1 |
|
NA - Sample not available for testing. |
Of the 176 saliva specimens collected from 88
infants, total salivary IgA was detected in all samples and
rotavirus-specific salivary IgA was detected in 124 (70.4%) saliva
specimens, as compared to 151 (85.7%) rotavirus-specific IgA in paired
serum samples. In the 3 and 5 dose arm together, 25 infants
pre-vaccination and 27 infants post-vaccination had no detectable levels
of rotavirus-specific salivary IgA.
The rotavirus-specific salivary IgA and the salivary
IgA normalized to 1 mg of total IgA both correlated with serum IgA pre-
and post-vaccination in both arms of the infant vaccination study (Fig.
1). Despite a significant positive correlation, when seroconversion
or seroresponse were examined at the individual level, there was low
correlation between serum and salivary antibody responses. Among the 43%
infants with a >4-fold increase in either salivary or serum IgA, 29
(33%) had a serum response and 17 (19%) had a salivary response. When
rotavirus salivary IgA was not normalized with total IgA, 4 (5%) more
infants had a ³4-fold
increase in rotavirus-specific salivary IgA.
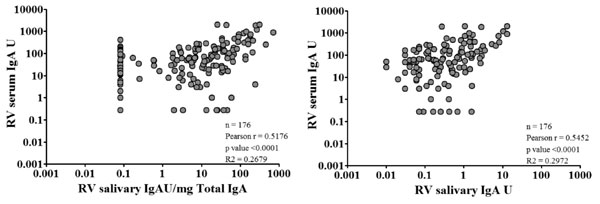 |
|
Fig. 1 Correlation between serum
anti-rotavirus IgA and salivary anti-rotavirus IgA antibodies.
|
Web Table I provides the GMC of serum,
salivary and fecal IgA stratified by serum response. Of the 88 infants,
13 had >4-fold IgA response in serum, saliva and fecal samples, 6 had a
2-4 fold increases in all specimens. Of the 35 infants who had no serum
immune response after vaccination, 15 infants showed increases in
rotavirus- specific salivary IgA levels and 13 infants also showed
measurable levels of fecal IgA. In this group of infants, the levels of
rotavirus-specific fecal IgA measured at 3 days post 1st dose of
vaccination showed a strong positive correlation with rotavirus specific
salivary IgA measured in secretions collected prior to vaccination
(r=0.96, P<0.0001). However, the fecal IgA measured at 3 days
post 3 or 5 dose of vaccination did not correlate with post vaccination
salivary IgA antibody levels.
Discussion
In early studies, we attempted to measure
rotavirus-specific IgA in parotid, sublingual secretions and whole
saliva and did not get satisfactory results. We subsequently used
improved collection devices along with a PBS-based oral-fluid transport
medium, which contains antibacterial and anti-proteolytic substances.
This oral fluid medium had previously been successfully used to study
salivary immune responses of rubella and pertussis vaccination and
infections [18,19]. We documented that rotavirus salivary IgA units
among adult volunteers were low but showed a significant positive
correlation with titres of rotavirus-specific serum IgA units. The
limited results from the adult vaccination study did not result in a
clear distinction of responses in parotid and non-parotid secretion. We
also documented that RV-salivary IgA/mg total IgA could be detected in
more than half of samples from infants who were vaccinated with
Rotavirus vaccine, but had negative results for serum antibodies. There
was a significant correlation between rotavirus-specific salivary and
serum IgA units, but seroresponse rates as fold changes between the pre-
and post-vaccination serum and salivary IgA of the infants did not
correlate perfectly, with salivary responses being generally lower than
serum.
Grimwood, et al. [14], showed that
anti-rotavirus IgA in saliva had a high predictive accuracy of almost
86% for specific IgA immune response in duodenal fluid of children at 4
weeks after rotavirus infection [5]. A report from Delhi [14] showed
that salivary IgA antibodies were a better indicator of asymptomatic
rotavirus infection in neonates than serum antibodies. The results from
this study suggest that measurement of salivary rotavirus IgA titers may
add to information on rotavirus infections and vaccine response.
However, it is important to note that previous studies [20,21] have
shown that both salivary and intestinal IgA levels rise and fall quickly
at about 2 weeks post infection or vaccination unlike serum antibodies
which peak at about day 27 post infection. Hence, collection of salivary
specimens at 2-week intervals might provide more valuable information to
answer whether salivary antibody levels actually reflect intestinal
antibody levels.
In our study, there was considerable variability in
the RV-fecal IgA responses of individual subjects. Bishop, et al.
[22], observed that among young infants, levels of fecal antibodies
fluctuated widely during the first few weeks of life while
breast-feeding was being established, possibly due to the amount of
breast milk ingested and intervals between evacuation of feces. Another
study speculated that the fluctuations in anti-rotavirus fecal antibody
levels reflects the fluctuating production in the small intestine, as a
response to a recurrent asymptomatic infection or to a persisting
infection [23].
Limitations of this study include a small sample
size, lack of more frequent sample collections and the absence of data
on factors such as stress and feeding that might affect salivary and
fecal IgA. Nonetheless, this is the first study to report evaluation of
IgA in serum and saliva in naturally exposed and vaccinated Indian
adults and infants and fecal IgA in vaccinated Indian infants. Given
that fecal IgA in particular has been described as a correlate of
protection in naturally infected children, such studies in vaccinated
children particularly in developing countries are needed. The results
indicate that evaluation of multiple samples is useful for intensive
experimental study designs and may help improve our understanding of the
induction and dynamics of immune responses to rotavirus vaccination.
Funding: The sponsor of the clinical trial for
adults was Shantha Biotechnics Limited. The sponsor of the clinical
trial with Rotarix was the Christian Medical College, Vellore. The
comparative immunogenicity study was supported by the Core Vaccine
Research Unit grant from the Department of Biotechnology, Government of
India BT/MB/VGCP/CVRU-CMC/2009 (GK). AP was supported by a Fogarty
International Center Global Infectious Disease Research training grant
D43 TW007392 (GK). The clinical trial sponsor had no role in the design
of the immunogenicity assessment, analysis of the data or preparation of
the manuscript.
Competing interest: None stated.
|
What This Study Adds?
• This study reports on evaluation of
rotavirus-specific IgA in serum and saliva in naturally exposed
and vaccinated Indian adults and infants and the first study to
report rotavirus specific fecal IgA in vaccinated Indian
infants.
|
References
1. Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon
I, Nagpal S, Goldberg E, et al. Vaccines for preventing rotavirus
diarrhoea: vaccines in use. Cochrane Database Syst Rev.
2012;11:CD008521.
2. de Oliveira LH, Camacho LA, Coutinho ES, Ruiz-Matus
C, Leite JP. Rotavirus vaccine effectiveness in Latin American and
Caribbean countries: A systematic review and meta-analysis. Vaccine.
2015;33:A248-54.
3. Karafillakis E, Hassounah S, Atchison C.
Effectiveness and impact of rotavirus vaccines in Europe, 2006-2014.
Vaccine. 2015;33:2097-107.
4. Vashishtha VM, Choudhury P, Kalra A, Bose A,
Thacker N, Yewale VN, et al. Indian Academy of Pediatrics (IAP)
recommended immunization schedule for children aged 0 through 18
years–India, 2014 and updates on immunization. Indian Pediatr.
2014;51:785-800.
5. Grimwood K, Lund JC, Coulson BS, Hudson IL, Bishop
RF, Barnes GL. Comparison of serum and mucosal antibody responses
following severe acute rotavirus gastroenteritis in young children. J
Clin Microbiol. 1988;26:732-8.
6. Franco MA, Angel J, Greenberg HB. Immunity and
correlates of protection for rotavirus vaccines. Vaccine.
2006;24:2718-31.
7. Jiang B, Gentsch JR, Glass RI. The role of serum
antibodies in the protection against rotavirus disease: an overview.
Clin Infect Dis. 2002;34:1351-61.
8. Davidson GP, Hogg RJ, Kirubakaran CP. Serum and
intestinal immune response to rotavirus enteritis in children. Infect
Immun. 1983;40:447-52.
9. Parry JV, Perry KR, Mortimer PP. Sensitive assays
for viral antibodies in saliva: An alternative to tests on serum.
Lancet. 1987;2:72-5.
10. Bishop R, Lund J, Cipriani E, Unicomb L, Barnes
G. Clinical serological and intestinal immune responses to rotavirus
infection of humans. Med Virol. 1990;9:85-109.
11. Riepenhoff-Talty M, Bogger-Goren S, Li P, Carmody
PJ, Barrett HJ, Ogra PL. Development of serum and intestinal antibody
response to rotavirus after naturally acquired rotavirus infection in
man. J Med Virol. 1981;8:215-22.
12. Brandtzaeg P. Do salivary antibodies reliably
reflect both mucosal and systemic immunity? Ann NY Acad Sci.
2007;1098:288-311.
13. Friedman M, Segal B, Zedaka R, Sarov B, Margalith
M, Bishop R, et al. Serum and salivary responses to oral
tetravalent reassortant rotavirus vaccine in newborns. Clin Exp Immunol.
1993;92:194-9.
14. Jayashree S, Bhan M, Kumar R, Raj P, Glass R,
Bhandari N. Serum and salivary antibodies as indicators of rotavirus
infection in neonates. J Infect Dis. 1988:1117-20.
15. Kompithra RZ, Paul A, Manoharan D, Babji S,
Sarkar R, Mathew LG, et al. Immunogenicity of a three dose and
five dose oral human rotavirus vaccine (RIX4414) schedule in south
Indian infants. Vaccine. 2014;32:A129-A33.
16. Paul A, Babji S, Sowmyanarayanan T, Dhingra MS,
Ramani S, Kattula D, et al. Human and bovine rotavirus strain
antigens for evaluation of immunogenicity in a randomized, double-blind,
placebo-controlled trial of a single dose live attenuated tetravalent,
bovine-human-reassortant, oral rotavirus vaccine in Indian adults.
Vaccine. 2014;32:3094-100.
17. Ward RL, Bernstein DI, Smith VE, Sander DS, Shaw
A, Eiden JJ, et al. Rotavirus immunoglobulin a responses
stimulated by each of 3 doses of a quadrivalent human/bovine reassortant
rotavirus vaccine. J Infect Dis. 2004;189:2290-3.
18. Litt DJ, Samuel D, Duncan J, Harnden A, George
RC, Harrison TG. Detection of anti-pertussis toxin IgG in oral fluids
for use in diagnosis and surveillance of Bordetella pertussis infection
in children and young adults. J Medical Microbiol. 2006;55:1223-8.
19. Nokes D, Enquselassie F, Vyse A, Nigatu W, Cutts
F, Brown D. An evaluation of oral-fluid collection devices for the
determination of rubella antibody status in a rural Ethiopian community.
Trans Royal SocTropMedHyg. 1998;92:679-85.
21. Kerr AC. The physiological regulation of salivary
secretions in man: A study of the response of human salivary glands to
reflex stimulation: Pergamon Press; 1961.
20. Bernstein DI, McNeal MM, Schiff GM, Ward RL.
Induction and persistence of local rotavirus antibodies in relation to
serum antibodies. J Med Virol. 1989;28:90-5.
21. Ward RL, Pax KA, Sherwood JR, Young EC, Schiff
GM, Bernstein DI. Salivary antibody titers in adults challenged with a
human rotavirus. J Med Virol. 1992;36:222-5.
22. Bishop RF, Bugg HC, Masendyez PJ, Lund JS,
Gorrell RJ, Barnes GL. Serum, fecal, and breast milk rotavirus
antibodies as indices of infection in mother-infant pairs. J Infect Dis.
1996;17:S22-S9.
23. Matson DO, O’Ryan ML, Herrera I, Pickering LK,
Estes MK. Fecal antibody responses to symptomatic and asymptomatic
rotavirus infections. J Infect Dis. 1993;167:577-83.
|
|
|
 |
|

