|
|
|
Indian Pediatr 2016;53: 589-593 |
 |
Rotavirus Infections in
Children Vaccinated Against Rotavirus in Pune, Western India
|
|
Preeti Jain, Gopalkrishna Varanasi, Rohan Ghuge,
*Vijay Kalrao, #Ram
Dhongade, $Ashish
Bavdekar, ‡Sanjay
Mehendale and Shobha Chitambar
From Enteric Viruses Group, National Institute of
Virology, Pune; *Bharati Vidyapeeth Medical College and Hospital, Pune;
#Sant Dnyaneshwar Medical Foundation & Research Centre’s
Shaishav Clinic, Pune; $King Edward Memorial Hospital, Pune;
and ‡National Institute of Epidemiology, Chennai; India.
Correspondence to: Dr Shobha D Chitambar, Enteric
Viruses Group, National Institute of Virology, 20-A, Ambedkar Road, PO
Box No 11, Pune 411 001, India.
Email: [email protected]
|
Objective: To characterize rotavirus infections detected in
rotavirus vaccinated children hospitalized for acute gastroenteritis.
Design: Observational, hospital-based study.
Setting: Three hospitals in Pune, Western India.
Participants: Children aged <5 years hospitalized
for acute gastroenteritis during 2013-14.
Methods: Rotavirus capture ELISA was performed on
all stool samples that were collected from patients following informed
consent from parents. VP7 and VP4 genes of rotavirus strains were
genotyped by multiplex RT-PCR. Stool samples from vaccinated children
were tested for other enteric viruses.
Results: Among the 529 children, 53 were
vaccinated with at least one dose of the rotavirus vaccine. There was no
difference in the mean (SD) (months) age of vaccinated [14.8 (10.6)] and
unvaccinated [14.4 (10.5)] children. Rotavirus positivity was
significantly higher (47%) in unvaccinated than in vaccinated (28.3%)
children (P=0.01). Mean Vesikari score and severe cases were
significantly more in rotavirus positive than in negative children
within unvaccinated group (P<0.001), while these did not differ
within the vaccinated group. Rotavirus strain G1P[8] was identified as
the most prevalent strain in both, vaccinated (60%) and unvaccinated
(72.8%) groups. No association was found between mean Vesikari score and
viral coinfections.
Conclusions: This study suggests decline in
rotavirus positivity in rotavirus-vaccinated children hospitalized for
acute gastroenteritis and high prevalence of G1P[8] and non-rotaviral
co-infections in Pune, Western India.
Keywords: Epidemiology, Diarrhea, Rotavirus vaccine.
|
|
Rotaviruses continue to be the commonest cause of
childhood acute gastroenteritis resulting in an estimated 24 million
outpatients visits, 2.5 million hospitalizations and 450,000 deaths
among children below 5 years of age worldwide [1]. Two rotavirus
vaccines, Rotarix, a monovalent human rotavirus vaccine and RotaTeq, a
pentavalent bovine-human reassortant vaccine have been licensed for use
in several countries including India [2, 3]. With evidence of their high
efficacy and safety in Americas, Europe, Australia and also in low
income countries, World Health Organization (WHO) has recommended their
inclusion in primary immunization programs globally [4,5]. Although
studies to assess immunogenicity and safety of Rotarix and RotaTeq have
been conducted successfully in India [6, 7], they have not yet been
introduced in the national immunization program [8]. However, their use
has been recommended by the Committee on Immunization in Children of the
Indian Academy of Paediatrics [9]. In a representative survey, routine
administration of rotavirus vaccine has been reported to be 9.7% by the
sampled pediatricians of India in 2009-2010 [10].
During the hospital-based rotavirus surveillance
being conducted at National Institute of Virology, Pune, Western India
among children who were hospitalized with acute gastroenteritis in the
years 2013-2014, we compared the demographic and clinical
characteristics, disease severity and virological status of children who
gave the history of rotavirus vaccination and others who did not.
Methods
The criteria for enrolment of children with acute
gastroenteritis and methodology used for clinical assessment and sample
collection have been described earlier [11]. Accordingly, children aged
less than 5 years of age admitted for acute gastroenteritis in three
different hospitals in Pune, India, were enrolled in the study.
Demographic and clinical data inclusive of age, date of onset and sample
collection, duration and maximum number of episodes of diarrhea and
vomiting, signs of dehydration, treatment and outcome of infection were
recorded for all patients. Severity of diarrhea was scored on the basis
of Vesikari scale [12]. Additionally, history of receipt of rotavirus
vaccine was obtained from children enrolled in the study. Efforts were
made to obtain accurate rotavirus vaccination history by referring to
their vaccination records and making phone calls to note the number of
doses, dates of administration and type of the rotavirus vaccine. Stool
samples were collected in sterile screw capped plastic containers and
transported on ice to the laboratory of National Institute of Virology,
Pune for detection of rotavirus antigen and strain characterization.
Prior to the enrolment of a child in the study, informed consent was
obtained from parent(s) or guardian. The study was approved by the
institutional and hospital Ethics Committees.
Ten percent (w/v or v/v) suspension was prepared in
0.01M phosphate buffered saline (PBS) pH 7.2 from all stool samples.
ELISA was performed on all suspensions using commercially available kit
(Premier Rotaclone, Meridian
Bioscience, Inc. USA) as per manufacturer’s instructions to detect the
presence of rotavirus antigen. The viral nucleic acids were extracted
from 30% (w/v) suspensions of all ELISA positive stool samples using
Trizol (Invitrogen, Carlsbad, CA) as per the manufacturer’s
instructions. The VP7 and VP4 genes were genotyped by multiplex reverse
transcription polymerase chain reaction (RT PCR) according to the
methods described earlier [13-15]. To determine the VP7 and VP4
genotypes of rotavirus strains non-typeable in multiplex PCR, first
round PCR products were sequenced using ABI-PRISM Big Dye Terminator
Cycle Sequencing Kit (Applied Biosystems, Foster city, CA) and a
ABI-PRISM 310 Genetic analyzer (Applied Biosystems) after purification
on minicolumns (QIAquick: Qiagen, Valencia, CA).
The presence of noro virus (NoV), enteric adeno virus
( AdV), human astro virus (HAst V) and entero virus (EV) was detected in
the stool specimens collected only from rotavirus-vaccinated children by
amplification of RdRp region A (126 bp), hexon (300 bp) ORF 1a (289 bp)
and 5’NCR (404 bp) regions, respectively as described earlier [16-19].
Statistical analysis: Two proportions were
compared using chi square test, two means were compared using
Mann-Whitney test and disease severity was compared by using chi square
test for 4X2 contingency table. P<0.05 were considered
statistically significant.
Results
Five hundred and twenty nine patients hospitalized
for acute gastroenteritis (from January 2013 to December 2014) at three
hospitals in Pune included 53 children (10%) who had received rotavirus
vaccine and 476 (90%) who had not. Among the vaccinated children, 18.9%,
56.6%, and 24.5% were administered with 3, 2 and 1 doses, respectively
of either of the monovalent (Rotarix) or pentavalent (RotaTeq) vaccines.
Male to female ratio (1.65:1 vs 1.64:1) and their age
distribution (0-6, 7-12, 13-18, 19-24, 25-59 months) were similar in
both groups. The mean (SD) age in months of vaccinated [14.8 (10.6)] and
unvaccinated [14.4 (10.5)] children hospitalized with acute
gastroenteritis was comparable.
Rotavirus positivity was found to be significantly
low (28.3%) in rotavirus vaccine recipients as compared to that of the
non-recipients (47%) (P=0.01) and was higher in males as compared
to females in both groups (P<0.05). The mean (SD) age (mo) in
rotavirus positive children in vaccinated and unvaccinated groups was
comparable [14.9 (9.2) and 13.5 (7.8)] (P>0.05). The same two
groups of rotavirus positive children also showed similar clinical
profile and presentation in terms of fever, history of vomiting and
diarrhea, duration of hospital stay and Vesikari scores (P>0.05)
(Table I).
TABLE I Characteristics of Rotavirus Vaccinated and Unvaccinated Children With and Without Rotavirus Gastroenteritis
|
Variables |
Vaccinated group |
Unvaccinated group |
|
Positive |
Negative |
P value |
Positive |
Negative |
P value |
|
Number |
15 |
38 |
- |
224 |
252 |
- |
|
Gender- Male; n (%) |
9 (60) |
24 (63.2) |
0.83 |
140 (62.5) |
156 (61.9) |
0.89 |
|
Age (mo), Mean (SD) |
14.9 (9.5) |
14.7 (11.1) |
0.79 |
13.5 (7.8) |
15.2 (12.4) |
0.62 |
|
Fever (≥37.10C), Mean
(SD) |
38.2 (0.3) |
38.1 (0.6) |
0.67 |
38.1 (0.6) |
38.3 (0.7) |
0.06 |
|
Vomiting |
|
Present: No. (%) |
14 (93.3) |
20 (52.6) |
0.005 |
205 (91.5) |
179 (71) |
<0.001 |
|
Duration (d), Mean (SD) |
1 .79 (1.1) |
1.65 (1.2) |
0.6 |
1.97 (1.0) |
2.16 (1.3) |
0.30 |
|
Episodes/day, Mean (SD) |
6.64 (5.0) |
4.7 (2.8) |
0.3 |
5.24 (3.6) |
4.13 (2.7) |
0.001 |
|
Diarrhea |
|
Duration (d), Mean (SD) |
2.33 (1.0) |
2.26 (1.2) |
0.64 |
2.19 (1.1) |
2.38 (1.1) |
0.13 |
|
Episodes/day, Mean (SD) |
13.13 (4.7) |
8.18 (3.7) |
0.001 |
11.14 (5.5) |
9.22 (5.2) |
<0.001 |
|
Hospital stay (d), Mean (SD) |
3.6 (1.1) |
4.4 (1.6) |
0.19 |
4.07 (1.1) |
4.63 (2.4) |
0.001 |
|
Vesikari score, Mean (SD) |
11.9 (2.4) |
10.5 (3) |
0.11 |
12.3 (2.0) |
11.4 (2.7) |
<0.001 |
|
Disease severity, No. (%) |
|
Mild |
0 |
1 (2.6) |
|
1 (0.4) |
3 (1.2) |
|
|
Moderate |
3 (20) |
18 (44.7) |
0.129 |
35 (15.6) |
89 (35.3) |
<0.001* |
|
Severe |
12 (80) |
17 (44.7) |
|
186 (83) |
145 (57.5) |
|
|
Very severe |
0 |
2 (5.3) |
|
2 (0.9) |
15 (6.0) |
|
Within the group of children who had not received
rotavirus vaccine, mean episodes of vomiting and diarrhea per day and
mean sikari scores were more commonly observed among rotavirus
positive than in rotavirus negative children (P=0.001, P<0.001
and P<0.001, respectively), and duration of hospital stay was
longer in rotavirus negative children than in rotavirus positive
children (P=0.001). Such analysis performed within the vaccinated
group of children showed more occurrences of vomiting and more number of
diarrheal episodes in rotavirus positive than in negative children (Table
I).).
The multiplex PCR performed on rotavirus positive
stool samples showed amplification of VP7 and VP4 genes in 93.3% and
100% of the strains, respectively from vaccinated group and in 98.8% and
98.3%, respectively from unvaccinated group. Among the vaccinated and
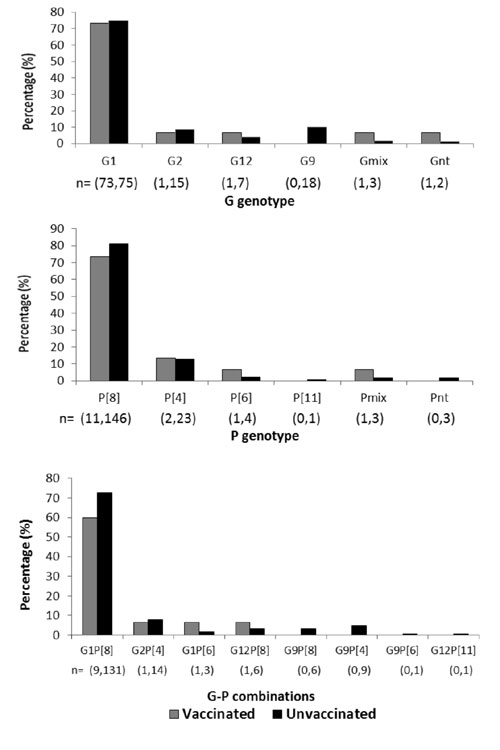
unvaccinated groups of children, the most prevalent G and P types were
G1 (73.3% and 75%) and P[8] (73.3% and 81.1%), respectively. Other
genotypes G2, G9, G12, P[4], P[6] and P[11] were detected at low levels
(0 % -13.3%) (Fig. 1a, 1b). Among the
strains typed for both genes, G1P[8] strains were detected at the
highest level in both (60% in vaccinated and 72.85 in unvaccinated)
groups. Frequency of detection of other common or unusual strains that
included G2P[4], G1P[6], G12P[8], G9P[8], G9P[4], G9P[6], and G12P[11]
ranged between 0.0% and 7.8 % (Fig. 1c). Overall
mixed infections of G and P genotypes (G1G9P[4], G1P[6]P[8], G1G9P[8],
G1G9P[4] P[8], G1G2P[8], G2P[4]P[8], and G9P[6]P[8]) were detected in
2.7%-13.3% children in both groups. Only 0.5% of the strains from the
unvaccinated group remained non-typeable for both genes.
 |
|
Fig. 1 Distribution of (a) G genotypes
(b) P genotypes and (c) common and unusual G-P combinations in
RVA strains detected in vaccinated and unvaccinated children.
The values in parentheses indicate respectively the number of
children in vaccinated and unvaccinated groups positive for a
particular genotype /G-P combination. Abbreviations - mix and nt
stand for mixed infections and non-typeables, respectively.
Gmix are G1/G2/G9 and Pmix are
P[4]/P[6]/P[8].
|
Stool samples from both rotavirus positive and
negative children from vaccinated group were examined for infections
with other enteric viruses (NoV, AdV, HAstV and EV) known to cause acute
gastroenteritis. Of the 53 children, 24 (45.2%) showed excretion of
single or multiple viruses in the stool. Single infections of NoV, AdV,
AstV and EV were detected in 5.6%, 11.3%, 1.8% and 13.2% respectively.
Mixed infections of rotavirus and other enteric viruses (NoV/ EV /HAstV)
were detected in six children (11.3%). In addition, mixed infections,
one each of EV with HAstV and AdV with HAstV were also detected.
Discussion
The present study reports the characteristics of
rotavirus infections in rotavirus vaccinated and unvaccinated children,
<5 years of age, hospitalized for acute gastroenteritis in Pune, Western
India during 2013-2014. The data of this study indicated that although
the rate of rotavirus vaccination among the enrolled children was only
10%, the vaccine recipients were less likely to have
rotavirus-associated gastroenteritis as compared to the non-recipients.
Further, similar disease severity scores and duration of hospital stay
were recorded in rotavirus positive and negative children in the
vaccinated group. In the unvaccinated group, significantly more severity
score was found in the rotavirus positive as compared to the rotavirus
negative children. In this (unvaccinated) group, longer duration of
hospital stay was noted in the rotavirus negative as compared to the
rotavirus positive children.
Most of the rotavirus-infected children from
unvaccinated group of the present study were below 2 years of age, and
the infections were clustered in post monsoon season as documented
earlier [20]. It has been reported that shifts in the average age and
seasonality pattern of rotavirus disease might take place in post
vaccination period [21]. In the present study, no change in the pattern
of rotavirus infections with respect to specific age and season (data
not shown) was noted in the vaccinated group. Although this finding may
be attributed to incomplete dosage and/or improper schedule followed for
vaccination, the interpretation has limitation due to presence of small
number of children in the vaccinated group.
Earlier reports from other countries have described
rise in the circulation of strains other than the vaccine strains after
the introduction of mono or pentavalent vaccines [22]. Virological
analysis of rotavirus positive stools performed in this study for both
vaccinated and unvaccinated groups was in agreement with the data
reporting diversity in circulating rotavirus strains [23] and
highlighted predominance of G1P [8] strains among both groups. It may be
noted that circulation of diverse rotavirus strains of a single genotype
has been reported continuously in children with acute gastroenteritis
from Pune and other regions of India [24,25]. On this backdrop,
infections with G1P [8] strains need to be delineated by analysis of all
capsid and internal genes. Further, to determine the vaccine induced
pressure on genotypes, it would be necessary to have higher coverage of
vaccination.
Evidence for the field effectiveness of rotavirus
vaccine(s) is highly desired to enhance its eventual country-wide
acceptance and use in the newer target populations. However, current
unavailability of this vaccine in the national immunization program and
its availability at comparatively high cost in the open market in India
restrict its usage as described earlier [10]. Although, the study
findings provide evidence of less likelihood of rotavirus infections in
vaccinated children, it is to be noted that in this study, extent of
vaccination coverage was variable and that the analysis was limited to
the small number of vaccine recipients who represented city based
pediatric population of Western India providing us less than the desired
power for detecting a significant difference in rotavirus disease
acquisition between the groups of vaccine recipients and non-recipients
(78%). However, the leads do indicate a promising role of the rotavirus
vaccine. In view of this, the observations made in this study need to be
ascertained by examination of a large number of rotavirus vaccine
recipients and non-recipients and eventually performing a classical
case-control study to more objectively prove the efficacy of rotavirus
vaccine in the Indian children. With rotavirus vaccine likely to be
rolled out in a step-wise manner in different states of India, a
systematic effort would be required to monitor the rotavirus infections
and genotypes in children presenting with rotavirus vaccine and
carefully document the rotavirus vaccine history to generate the data on
its effectiveness in different regions of country.
Contributors: PJ: Conducted the laboratory tests,
interpreted and analyzed the data and drafted the manuscript; GV:
Coordinated and organized collection of clinical data and laboratory
work; RG: Recorded demographic and clinical data; VK, RD and AB:
Conducted clinical examination of patients admitted to the hospitals;
SM: Coordinated and monitored the surveillance activity of Pune center
and contributed to manuscript development; SC: Conceived and designed
the study and revised the manuscript for important intellectual content.
The final manuscript was approved by all authors.
Funding: Indian Council of Medical
Research
Competing Interests: None stated.
Acknowledgements: Dr DT Mourya, Director,
NIV, Pune for his constant support and Mr Atul Walimbe for his
assistance in statistical analysis of the data. Technical assistance of
Ms. Kanak Borawake and Mr Vishal Jagtap is gratefully acknowledged.
|
What is Already Known?
• Rotavirus vaccines were found to be
immunogenic with good safety profile in studies conducted in
India.
What This Study Adds?
• Rotavirus positivity is significantly lesser in vaccinated
children admitted with diarrhea in comparison to non-vaccinated
children.
|
References
1. Tate JE, Burton AH, Boschi-Pinto C, Steele AD,
Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated
mortality in children younger than 5 years before the introduction of
universal rotavirus vaccination programmes: A systematic review and
meta-analysis. Lancet Infect Dis. 2012;12:136-41.
2. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR,
Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an
attenuated vaccine against severe rotavirus gastroenteritis. N Engl J
Med. 2006;354:11-22.
3. Vesikari T, Matson DO, Dennehy P, Van Damme P,
Santosham M, Rodriguez Z, et al. Safety and efficacy of a
pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J
Med. 2006;354:23-33.
4. American Academy of Pediatrics Committee on
Infectious Diseases. Prevention of rotavirus disease: Updated guidelines
for use of rotavirus vaccine. Pediatrics. 2009; 123:1412-20.
5. Rotavirus vaccines WHO position paper: January
2013 - Recommendations. Vaccine. 2013;31:6170-1.
6. Narang A, Bose A, Pandit AN, Dutta P, Kang G,
Bhattacharya SK, et al. Immunogenicity, reactogenicity and safety
of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccine.
2009;5:414-19.
7. Lokeshwar MR, Bhave S, Gupta A, Goyal VK, Walia A.
Immunogenicity and safety of the pentavalent human-bovine (WC3)
reassortant rotavirus vaccine (PRV) in Indian infants. Hum Vaccin
Immunother. 2013;9:172-6.
8. Vashishtha VM, Choudhury P, Kalra A, Bose
A, Thacker N, Yewale VN, et al. Indian Academy of Pediatrics
(IAP) recommended immunization schedule for children aged 0 through 18
years – India, 2014 and updates on immuni-zation. Indian Pediatr.
2014;51: 785-800.
9. Indian Academy of Pediatrics Committee on
Immunization (IAPCOI). Consensus recommendations on immunization, 2008.
Indian Pediatr. 2008;45:635-48.
10. Gargano LM, Thacker N, Choudhury P, Weiss PS,
Pazol K, Bahl S, et al . Predictors of administration and
attitudes about pneumococcal, Haemophilus influenzae type b and
rotavirus vaccines among pediatricians in India: A national survey.
Vaccine. 2012; 30:3541-5.
11. Kang G, Arora R, Chitambar SD, Deshpande J, Gupte
MD, Kulkarni M, et al. Multicenter, hospital-based surveillance
of rotavirus disease and strains among Indian children aged< 5 years. J
Infect Dis. 2009; 200:S147-S53.
12. Ruuska T, Vesikari T. A prospective study of
acute diarrhoea in Finnish children from birth to 2 1/2 years of age.
Acta Paediatr Scand. 1991;80:500-7.
13. Tatte VS, Gentsch JR, Chitambar SD.
Characterization of group A rotavirus infections in adolescents and
adults from Pune, India: 1993-1996 and 2004-2007. J Med Virol. 2010;
82:519-27.
14. Freeman MM, Kerin T, Jennifer H, Caustland KM,
Gentsch J. Enhancement of detection and quantification of rotavirus in
stool using a modified real time RT-PCR assay. J Med Virol.
2008;80:1489-96.
15. Gentsch JR, Glass RI, Woods P, Gouvea V,
Gorziglia M, Flores J, et al. Identification of group A rotavirus
gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;
30:1365-73.
16. Girish R, Broor S, Dar L, Ghosh D. Foodborne
outbreak caused by a Norwalk- like virus in India. J Med Virol.
2002;67:603-7.
17. Allard A, Girones R, Juto P, Wadell G. Polymerase
chain reaction for detection of adenoviruses in stool samples. J Clin
Microbiol. 1990;28:2659-67.
18. Noel JS, Lee TW, Kurtz JB, Glass RI, Monroe SS.
Typing of human astroviruses from clinical isolates by enzyme
immunoassay and nucleotide sequencing. J Clin Microbiol.
1995;33:797-801.
19. Sapkal GN, Bondre VP, Fulmali PV, Patil P,
Gopalkrishna V, Dadhania V, et al. Enteroviruses in patients with
acute encephalitis, Uttar Pradesh, India. Emerg Infect Dis.
2009;15:295-8.
20. Saluja T, Sharma SD, Gupta M, Kundu R, Kar S,
Dutta A, et al. A multicenter prospective hospital-based
surveillance to estimate the burden of rotavirus gastroenteritis in
children less than five years of age in India. Vaccine. 2014;32:A13-9.
21. Patel MM, Steele D, Gentsch JR, Wecker J, Glass
RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr
Infect Dis J. 2011;30:S1-S5.
22. Kirkwood CD, Boniface K, Barnes GL, Bishop RF.
Distribution of rotavirus genotypes after introduction of rotavirus
vaccines, Rotarix® and RotaTeq®, into the National Immunization Program
of Australia. Kirkwood CD, Boniface K, Barnes GL, Bishop RF. Pediatr
Infect Dis J. 2011;30:S48-53.
23. Kang G, Desai R, Arora R, Chitamabar S, Naik TN,
Krishnan T, et al. Diversity of circulating rotavirus strains in
children hospitalized with diarrhea in India, 2005-2009. Vaccine.
2013;31:2879-83.
24. Samajdar S, Ghosh S, Dutta D, Chawla-Sarkar M,
Kobayashi N, Naik TN. Human group A rotavirus P[8] Hun9-like and rare
OP354-like strains are circulating among diarrhoeic children in Eastern
India. Arch Virol. 2008;153:1933-6.
25. Kulkarni R, Arora R, Arora R, Chitambar, SD.
Sequence analysis of VP7 and VP4 genes of G1P [8] rotaviruses
circulating among diarrhoeic children in Pune, India: A comparison with
Rotarix and RotaTeq vaccine strains. Vaccine. 2014;32:A75-A83.
|
|
|
 |
|

