|
|
|
Indian Pediatr 2016;53: 583-588 |
 |
Rotavirus and other
Diarrheal Disease in a Birth Cohort from Southern Indian
Community
|
|
R Sarkar, BP Gladstone, JP Warier,
SL Sharma, U Raman, J Muliyil and G Kang
From Division of Gastrointestinal Sciences, and
*Community Health Department, Christian Medical College, Vellore, Tamil
Nadu, India.
Correspondence to: Dr Gagandeep Kang, Division of
Gastrointestinal Sciences, Christian Medical College,
Vellore 632 004,
Tamil Nadu, India.
Email: [email protected]
Received: May 15, 2015;
Initial review: October 09, 2015;
Accepted: May 05, 2016.
|
Objective: To describe the incidence, severity
and etiology of diarrheal disease in infants and young children residing
in an urban slum community in Southern India.
Setting: Three contiguous urban slums in Vellore,
Tamil Nadu.
Participants: 452 children participating
in a birth cohort study on diarrheal disease; 373 completed three years
of follow-up.
Outcome measures: Diarrheal incidence (obtained
by twice-weekly home visits) and severity (assessed by the Vesikari
scoring system), and etiological agents associated with diarrhea
(through examination of stool specimens by bacteriologic culture,
rotavirus enzyme immunoassay, PCR for norovirus and microscopy for
parasites).
Results: A total of 1856 diarrheal
episodes were reported in 373 children. The overall incidence rate of
diarrhea was 1.66 episodes per child year for three years, with 2.76
episodes per child year in infancy. The incidence peaked during the
months of July and August. Severe diarrhea formed 8% of the total
episodes. Rotavirus was the most common pathogen detected, being
identified in 18% of episodes. Good hygiene status resulted in 33%
protection against moderate-to-severe diarrhea.
Conclusions: This study highlights the burden of
diarrheal disease and the important etiological agents of childhood
diarrhea in Southern India. Promotion of hygienic behavior through
health education may help reduce diarrheal incidence in this and similar
communities.
Keywords: Birth cohort, Diarrhea, Children,
Epidemiology, Etiology.
|
|
A
cute diarrheal diseases are one of the top five
causes of morbidity and mortality worldwide, accounting for 0.52 million
deaths annually in children under the age of 5 years [1]. The most
common enteropathogens causing diarrhea are rotavirus,
Cryptosporidium spp., Salmonella spp., Campylobacter,
Shigella, diarrheagenic Escherichia coli, calicivirus,
adenovirus, astrovirus and possibly Giardia. Rotavirus, norovirus
and diarrheagenic Escherichia coli. are responsible for more than
half of all diarrheal deaths in under-five children [2].
While mortality is an important measure of disease
burden, a true estimate of the global impact includes outcomes from the
entire spectrum of mild, moderate and severe forms of disease. Although
the diarrhea-related mortality has decreased by 68% between 1990 and
2013 [1], the corresponding decline in disease incidence has been more
modest [3]. It has been estimated that approximately 35.2% of all
diarrheal episodes are moderate-to-severe in nature [4]; but there are
considerable uncertainties surrounding these estimates due to lack of
robust country-specific data, especially from regions with the highest
disease burden.
Given the importance of diarrheal disease as a cause
of both deaths and malnutrition in children [5,6], it is important to
have community-based data in order to derive a true estimate of the
national disease burden. This study reports incidence, clinical features
and etiology of diarrheal disease in a birth cohort in an urban slum
community in Southern India.
Methods
The recruitment and follow-up of the cohort has been
previously described [7]. In this cohort, 452 newborn infants were
recruited from three contiguous urban slums in Vellore between March
2002 and August 2003. They were followed up with twice weekly home
visits by field workers, who enquired about morbidity, until they
attained the age of three years. Stool samples were collected whenever a
child was found to have an episode of diarrhea. During a diarrheal
episode, the child was visited on alternate days until resolution.
Detailed clinical data was collected on the onset, duration, frequency,
color and consistency of stools, associated vomiting and fever, presence
and severity of dehydration, and treatment. The Institutional Review
Board of Christian Medical College, Vellore, approved the study and
written informed consent was obtained from parents/guardians of all
children prior to enrollment.
Diarrhea was defined as the passage of three or more
loose watery stools in a 24-hour period, or change in the number or
consistency of the stools. An episode of diarrhea
was defined as at least one day of diarrhea, preceded and followed by
two or more days without diarrhea [7]. A diarrheal episode was said to
be associated with a specific pathogen if the pathogen was isolated from
stool samples collected at the time of the episode or within a week
after the cessation of the episode. In case of rotavirus, it was within
a week before or after the actual period of the episode of diarrhea.
Acute diarrhea was defined as an episode lasting for less than 14
days and persistent diarrhea as an episode lasting for 14 days or
more [8].
Severity of diarrhea was assessed using the Vesikari
scale, originally designed for assessing rotavirus disease presenting to
hospital [9]. An episode was considered mild for a Vesikari score 5 or
less, moderate for 6-10 and severe for 11 or more. Data on baseline
socio-demographic characteristics, feeding patterns, and monthly
anthropometric (weight and height) measurements were recorded.
Height-for age (HAZ), weight-for-age (WAZ) and weight-for-height (WHZ)
z-scores were calculated using the 2006 WHO child growth standards as
the reference population [10]. Children with HAZ, WHZ and WAZ of <-2 SD
were considered to be stunted, wasted and underweight, respectively.
Hygiene status of the household was recorded for all
children at the time of recruitment and thereafter at 6-monthly
intervals, using a previously validated questionnaire [11], and each
household assigned a score ranging from 0 to 21. Households were then
classified as good ( ³13),
poor (10-12) and very poor (£9)
hygiene status based on their score.
Laboratory methods: Microscopy and culture of
stool specimens was done on the same day of collection using standard
methods to identify various enteropathogens causing diarrhea.
Enteropathogenic E. coli (EPEC) were identified by serogrouping
and other classes of diarrheagenic E. coli were not tested
[12]. We identified rotavirus in diarrheal stool by ELISA, but also
performed RNA extraction and RT-PCR assay even if the screening ELISA
was negative [13]. Noroviruses and sapoviruses, which belong to the
Caliciviridae family, were identified using PCR carried out on a
subset of 500 diarrheal samples [14]. Cryptosporidium spp. was
identified using modified acid-fast staining [15].
Statistical analysis: The overall and
pathogen-specific incidence rates of diarrhea episodes, season-specific
incidence, and severity and age at infection for specific diarrheal
pathogens were calculated using the number of episodes as the numerator
and the total child-years of follow-up as the denominator. Factors
influencing severity of diarrhea (moderate/severe vs. mild) was
assessed using logistic regression analysis and odds ratios with 95%
confidence intervals (CI) were calculated.
Results
A total of 1856 diarrheal episodes were documented in
373 children, who completed three years of follow-up. Stool samples were
collected for 1829 episodes (98.6%). The overall incidence rate of
diarrhea was 1.66 episodes per child-year, with the highest incidence of
2.76 episodes per child-year during infancy. During the second and third
years of life, diarrheal incidence was 1.28 and 0.94 episodes per
child-year, respectively.
Ninety-five percent of the children had at least one
episode, and 28% had more than 6 episodes of diarrhea by the time they
reached 3 years of age. By 4 months of age, 50% had at least one
episode, and at the end of 6 months, 75% had one or more episodes of
diarrhea. The highest incidence of diarrhea was observed in children
aged between 3 and 8 months.
Median (IQR) duration of a diarrheal episode was 3
(2-4) days. Duration varied with age (P<0.001), with 42% of
episodes in first year longer than 3 days, followed by 29% and 22% in
second and third years, respectively. A total of 1833 (98.8%) acute and
23 (1.2%) persistent diarrhea episodes were reported in our study.
Vomiting and fever accompanied 297 (16%) and 317
(17%) episodes, respectively. Dehydration, positively associated with
age (P<0.001), was seen in 191 (10.6%) episodes with the highest
prevalence during 24-30 months of age; majority of them (167, 87.4%)
were mild (1-5%) dehydration. Children were taken to an outpatient
clinic or a hospital during 1306 (72%) diarrheal episodes and 43 (2.4%)
needed hospitalization, of which 11 (0.6%) episodes needed intravenous
rehydration. Oral rehydration was given for 1564 (87.4%) episodes. Mucus
in stool was observed in 250 (13.5%) episodes whereas bloody diarrhea
was reported in 41 (2.2%) episodes. Antibiotics and antimotility drugs
were prescribed during 27.5% and 14.6% of the episodes, respectively.
Severity of diarrhea was assessed for 1793 (96.6%) episodes wherein
58.4% were mild, 33.4% moderate and 8.2% severe. Antibiotics were
prescribed for 19.1% of mild, 35.8% of moderate and 54.2% of severe
diarrheal episodes (P<0.001). The proportion of severe diarrhea
episodes was highest in first six months of life (12%) and decreased
subsequently.
Of the 1829 episodes of diarrhea for which stool
samples were available for analysis, one or more pathogens were isolated
from 635 (35.7%) episodes. Isolation of diarrheagenic microorganisms was
lower during infancy (28%) than during the later years (45%). Rotavirus
was detected in 18% of stool samples, followed by Giardia (8%),
Aeromonas (4%), Cryptosporidium (3%), Shigella (2%)
and Vibrio cholerae (1%). The pathogen-specific diarrheal
incidence is presented in Table I. Co-infection with two
pathogens was observed in 88 (4.8%) of diarrheal episodes and in 9
(0.5%) episodes, three or more pathogens were detected.
TABLE I Specific Pathogens Identified in 1829 Diarrheal Episodes
|
Pathogen detected in stool |
No. (%) |
Incidence rate |
|
of diarrheal |
per child year |
|
episodes |
(95% CI) |
|
Bacteria |
|
|
|
Aeromonas spp. |
69 (3.8) |
6.17 (4.72-7.63) |
|
Salmonella spp. |
8 (0.4) |
0.72 (0.22-1.21) |
|
Shigella spp. |
41 (2.2) |
3.67 (2.54-4.79) |
|
Vibrio cholerae |
18 (1.0) |
1.61 (0.87-2.35) |
|
Enteropathogenic |
9 (0.5) |
0.81 (0.28-1.33) |
|
E.coli |
|
|
|
Parasite |
|
|
|
Cryptosporidium spp. |
57 (3.1) |
5.1 (3.77-6.42) |
|
Giardia spp. |
148 (8.1) |
13.24 (11.11-15.37) |
|
Virus |
|
|
|
Rotavirus |
324 (17.7) |
28.98 (25.82-32.14) |
|
Norovirus* |
35 (7.0) |
3.13 (2.09-4.17) |
|
Sapovirus* |
18 (3.6) |
1.61 (0.87-2.35) |
|
* Number of samples tested = 500 |
Vibrio cholerae and EPEC infections occurred
mostly during infancy, whereas Giardia and Shigella tended
to occur later in life. Among viruses, rotavirus and calicivirus (norovirus
and sapovirus) had a mild preponderance during the first year of life.
The median (IQR) age at infection and severity scores for different
pathogens are presented in Table II. Vibrio-associated
episodes were found to be most severe. In general, diarrheal episodes
associated with viral pathogens [median (IQR) Vesikari score = 6 (5-9),
P<0.001] were found to be more severe than those associated with
bacterial [median (IQR) Vesikari score = 5 (5-7), P=0.156] or
parasitic [median (IQR) Vesikari score = 5 (4-7), P=0.221]
pathogens. Also, antibiotic usage was significantly associated with
viral (32.5% vs. 26.5%, P=0.027), but not bacterial (32.8%
vs. 27.3%, P=0.175) or parasitic (23.9% vs. 28.2%, P=0.222)
diarrhea.
TABLE II Age at Infection and Severity for Specific Pathogens Identified in
|
Pathogen detected in stool |
Age at infection |
Severity score |
|
n |
Median |
n |
Median |
|
|
(IQR) |
|
(IQR) |
|
Aeromonas spp. |
69 |
16 (10-23) |
69 |
5 (4-8) |
|
Salmonella spp. |
8 |
20 (10-24) |
5 |
5 (5-6.5) |
|
Shigella spp. |
41 |
18 (14-27) |
41 |
5(5-6) |
|
Vibrio cholerae |
18 |
11 (5-20) |
18 |
7 (5-13) |
|
Enteropathogenic Escherichia coli |
9 |
8 (6-11) |
8 |
5 (5-6) |
|
Cryptosporidium spp. |
54 |
14 (9-24) |
53 |
5 (4-7.5) |
|
Giardia spp. |
148 |
23 (16-28) |
146 |
5 (4-7) |
|
Rotavirus |
324 |
10 (4-17) |
209 |
6 (5-9) |
|
Norovirus* |
35 |
11 (8-11) |
35 |
5 (5-7) |
|
Sapovirus* |
18 |
14 (10-22) |
18 |
5 (4-8) |
|
*Number of samples tested = 500. |
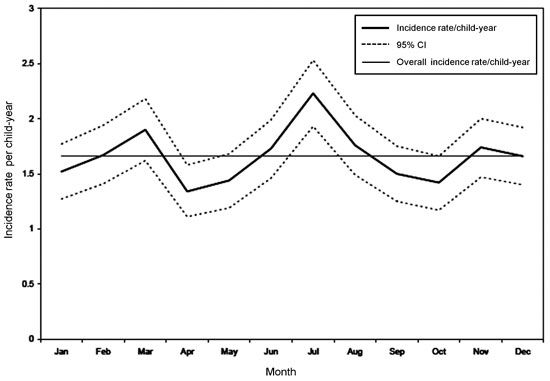
The highest peak in the incidence of diarrhea was
observed during the month of July and August (Fig. 1).
When individual pathogens were considered, Giardia peaked in
August while rotavirus showed two peaks, one in January followed by a
smaller peak in June. Cryptosporidium, on the other hand, showed
a small peak during the cooler months (January-March). Other pathogens
did not demonstrate any seasonal variation.
 |
|
Fig. 1 Seasonal pattern of diarrheal
incidence among children in the birth cohort, followed from
March 2002 to August 2006.
|
There was no association between diarrhea and
breastfeeding or nutritional status, while wasting showed a weak
evidence of being a risk factor for moderate/severe diarrhea (OR =
1.26). Household hygiene was positively associated with diarrheal
severity, with children from households with poor and very poor hygiene
having a 46% and 49% elevated risk of suffering from moderate/severe
diarrhea, respectively, as compared to those belonging to families with
good hygiene (Table III).
TABLE III Association of Breastfeeding and Nutritional Status with Severity of Diarrhea (n=373)
|
Exposure status |
Diarrheal episodes |
|
Mild, No. (%) |
Moderate/severe, No. (%) |
Odds ratio (95% CI)* |
|
Exclusive breastfeeding at the time of diarrhea |
137 (12) |
95 (12) |
1.04 (0.76-1.41) |
|
Any breastfeeding at the time of diarrhea |
682 (61) |
468 (63) |
1.06 (0.85-1.32) |
|
Wasted (WHZ<-2 SD)# |
181 (16) |
147 (20) |
1.26 (0.94-1.69) |
|
Stunted (HAZ<-2 SD)# |
493 (44) |
317 (42) |
0.93 (0.75-1.14) |
|
Underweight (WAZ<-2 SD)# |
365 (33) |
250 (33) |
1.03 (0.81-1.31) |
|
Malnourished (wasted/stunted/underweight)# |
460 (41) |
327 (44) |
0.91 (0.73-1.12) |
|
Hygiene status§ |
|
|
|
|
Good |
429 (39) |
222 (30) |
1¶ |
|
Poor |
290 (26) |
222 (30) |
1.48 (1.15-1.93) |
|
Very poor |
391 (35) |
302 (40) |
1.49 (1.13-1.97) |
|
*Estimates adjusted for repeated episodes per child; #Calculations
based on measurement within a month prior to each diarrheal
episode; §Calculations based on
hygiene status assessed at 6 months of age; ¶Reference
category. |
Discussion
The epidemiology of childhood diarrhea in this
southern Indian urban slum showed an incidence rate of 1.66 episodes per
child-year. The estimate of diarrheal incidence in our study was close
to that of 1.69 episodes per child-year among under-five children in a
Delhi urban slum [16], but was below the median global estimate of 2.9
episodes [3]. In concordance with what has previously been reported [3],
the diarrheal incidence peaked during infancy, decreasing steadily
thereafter. This peak in incidence during infancy is possibly due to the
consumption of foods that are improperly cooked or are prepared under
unhygienic conditions [17].
The majority (72%) of the diarrheal episodes in our
study resulted in a visit to a doctor, which can be attributed to the
free and easy access to health care. The usage of oral rehydration
solution (ORS) was also much higher compared to 32% in Tamil Nadu [18]
and 39% in a Delhi urban slum [16], which can be attributed to the
intensive supervision by the field worker and doctors’ advice during
each clinic visit. On the other hand, high usage of antimotility and
antibiotic drugs was also noticed in this study, which contrasts with
the national [19] and international [8] guidelines that recommend
against the use medications for childhood diarrhea, except ORS for
dehydration, and antibiotics only for certain culture-proven infections.
Increased use of antibiotics for the treatment of diarrhea in Indian
children has been reported earlier [20].
In this study, diarrheagenic microorganisms could be
isolated from only 35.7% of all reported episodes of diarrhea. Similar
findings have also been reported from other population-based studies
[21,22]. The proportion of diarrheal episodes from which any pathogen
could be isolated was much lower in first year (28%) than the next two
years (45%), possibly due to transitional diarrhea of the newborn or
weaning diarrhea. Transitional diarrhea can occur as a result of failure
of adaptation to enteral feeding and microbial colonisation during the
weaning period [23]. Other potential reasons for the large proportion of
unaccounted diarrheal episodes are low pathogen yield due to delay in
sample collection [24], intermittent pathogen shedding [25], lower
sensitivity of conventional diagnostic methods for the detection of
stool parasites [26], and the presence of novel diarrheagenic pathogens
[27].
An earlier study from Manipal, on hospitalized
children with diarrhea showed rotavirus in 5.2%, Salmonella in
5.9%, Shigella in 5.4%, Aeromonas in 4.1% and Vibrio
in 1.3% of the diarrheal samples [28]. In a multi-centric
community-based study on the etiology of acute diarrhea, rotavirus,
Cryptosporidium spp., enterotoxigenic Escherichia coli and
Shigella were the major causes of moderate-to-severe diarrhea among
children in developing countries [29]. In our study too, rotavirus,
Shigella and Cryptosporidium spp. were the commonest
pathogens isolated from diarrheal stool samples, thereby highlighting
the importance of these pathogens in causing childhood diarrhea.
In our analysis, poor and very poor hygiene status
showed a higher risk of getting severe diarrhea. This is in confirmation
with studies done in other parts of India which have shown that good
hygiene practices within the home, such as washing hands with soap
before feeding a child, can reduce the risk of childhood diarrhea [30].
In conclusion, this study provides a better
understanding of the etiology of childhood diarrhea in a community
setting, besides increasing our awareness about the unnecessary usage of
antibiotic and antimotility drugs. Similar to what is observed in
hospitalized children, rotavirus was the commonest etiological agent
associated with childhood diarrhea in this community. Health education
to promote hygiene behavior can be an effective low cost intervention to
reduce the incidence of severe diarrhea.
Acknowledgements: The authors thank the
field workers and the support staff of the Wellcome Trust Research
Laboratory at CMC, Vellore. They also thank Dr. Shobhana and the
municipal health team for their help with recruitment, and Dr. Anuradha
Bose for looking after the children who were admitted to CHAD hospital.
Contributors: JM and GK: conceived and designed
the study, and revised the manuscript for important intellectual
content; BPG, JPW, SLS and UR: conducted the study and helped in
manuscript writing; RS and BPG: analyzed the data and drafted the
manuscript. All authors approved the final version of the manuscript; RS
and GK: are guarantors of the paper.
Funding: Wellcome Trust under the Trilateral
Cooperative initiative for Research in Infectious Diseases in the
Developing World (Grant number: 063144 to GK). RS was supported by the
Fogarty International Center Global Infectious Disease Research Training
Program (Grant number: D43 TW007392 to GK). Competing Interests:
None stated.
|
What is Already Known?
• The etiology of diarrhea in hospitalized
children is well documented, but community estimates from
longitudinal studies are lacking.
What This Study Adds?
• Incidence rate of diarrhea was 1.66
episodes per child year for first 3 years of life; highest
incidence (2.76 episodes per child year) is seen in infancy.
• As with hospitalized children, rotavirus
was the commonest etiological agent associated with childhood
diarrhea in the community.
|
References
1. GBD 2013 Mortality and Causes of Death
Collaborators. Global, regional, and national age–sex specific all-cause
and cause-specific mortality for 240 causes of death, 1990–2013: A
systematic analysis for the Global Burden of Disease Study 2013. Lancet
2014;385:117-71.
2. Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres
CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in
children <5 years of age: A systematic review. PLoS One. 2013;8:e72788.
3. Fischer Walker CL, Perin J, Aryee MJ, Boschi-Pinto
C, Black RE. Diarrhea incidence in low- and middle-income countries in
1990 and 2010: A systematic review. BMC Public Health. 2012;12:220.
4. Lamberti LM, Fischer Walker CL, Black RE.
Systematic review of diarrhea duration and severity in children and
adults in low- and middle-income countries. BMC Public Health.
2012;12:276.
5. Mata L. Diarrheal disease as a cause of
malnutrition. Am J Trop Med Hyg. 1992;47:16-27.
6. Liu L, Johnson HL, Cousens S, Perin J, Scott S,
Lawn JE, et al. Global, regional, and national causes of child
mortality: An updated systematic analysis for 2010 with time trends
since 2000. Lancet. 2012;379:2151-61.
7. Gladstone BP, Muliyil JP, Jaffar S, Wheeler JG, Le
Fevre A, Iturriza-Gomara M, et al. Infant morbidity in an Indian
slum birth cohort. Arch Dis Child. 2008;93:479-84.
8. WHO. The Treatment of diarrhoea: A Manual for
Physicians and Other Senior Health Workers. Geneva: World Health
Organization; 2005.
9. Ruuska T, Vesikari T. Rotavirus disease in Finnish
children: Use of numerical scores for clinical severity of diarrhoeal
episodes. Scand J Infect Dis. 1990;22:259-67.
10. WHO. WHO Child Growth Standards:
Length/Height-for-Age, Weight-for-Age, Weight-for-Length,
Weight-for-Height and Body Mass Index-for-Age: Methods and Development.
Geneva: World Health Organization; 2006.
11. Brick T, Primrose B, Chandrasekhar R, Roy S,
Muliyil J, Kang G. Water contamination in urban south India: Household
storage practices and their implications for water safety and enteric
infections. Int J Hyg Environ Health. 2004;207:473-80.
12. Rajendran P, Ajjampur SS, Chidambaram D,
Chandrabose G, Thangaraj B, Sarkar R, et al. Pathotypes of
diarrheagenic Escherichia coli in children attending a tertiary care
hospital in South India. Diagn Microbiol Infect Dis. 2010;68:117-22.
13. Banerjee I, Ramani S, Primrose B, Moses P,
Iturriza-Gomara M, Gray JJ, et al. Comparative study of the
epidemiology of rotavirus in children from a community-based birth
cohort and a hospital in South India. J Clin Microbiol. 2006;44:2468-74.
14. Monica B, Ramani S, Banerjee I, Primrose B,
Iturriza-Gomara M, Gallimore CI, et al. Human caliciviruses in
symptomatic and asymptomatic infections in children in Vellore, South
India. J Med Virol. 2007;79:544-51.
15. Ajjampur SS, Gladstone BP, Selvapandian D,
Muliyil JP, Ward H, Kang G. Molecular and spatial epidemiology of
cryptosporidiosis in children in a semiurban community in South India. J
Clin Microbiol. 2007;45:915-20.
16. Gupta N, Jain SK, Chawla U, Hossain S, Venkatesh
S. An evaluation of diarrheal diseases and acute respiratory infections
control programmes in a Delhi slum. Indian J Pediatr. 2007;74:471-6.
17. Motarjemi Y, Kaferstein F, Moy G, Quevedo F.
Contaminated weaning food: A major risk factor for diarrhoea and
associated malnutrition. Bull World Health Organ. 1993;71:79-92.
18. International Institute for Population Sciences
(IIPS) and Macro International. National Family Health Survery (NFHS-3),
India, 2005-06: Tamil Nadu. Mumbai: IIPS; 2008.
19. Bhatnagar S, Lodha R, Choudhury P, Sachdev HP,
Shah N, Narayan S, et al. IAP Guidelines 2006 on management of
acute diarrhea. Indian Pediatr. 2007;44:380-9.
20. Kotwani A, Chaudhury RR, Holloway K.
Antibiotic-prescribing practices of primary care prescribers for acute
diarrhea in New Delhi, India. Value Health. 2012;15:S116-9.
21. de Wit MA, Koopmans MP, Kortbeek LM, Wannet WJ,
Vinje J, van Leusden F, et al. Sensor, a population-based cohort
study on gastroenteritis in the Netherlands: Incidence and etiology. Am
J Epidemiol. 2001;154: 666-74.
22. Valentiner-Branth P, Steinsland H, Fischer TK,
Perch M, Scheutz F, Dias F, et al. Cohort study of Guinean
children: Incidence, pathogenicity, conferred protection, and
attributable risk for enteropathogens during the first 2 years of life.
J Clin Microbiol. 2003;41:4238-45.
23. Maiya PP, Jadhav M, Albert MJ, Mathan M.
Transitional diarrhoea in newborn infants. Ann Trop Paediatr.
1985;5:11-4.
24. Tarr PI, Neill MA, Clausen CR, Watkins SL,
Christie DL, Hickman RO. Escherichia coli O157:H7 and the hemolytic
uremic syndrome: importance of early cultures in establishing the
etiology. J Infect Dis. 1990;162:553-6.
25. Mukhopadhya I, Sarkar R, Menon VK, Babji S, Paul
A, Rajendran P, et al. Rotavirus shedding in symptomatic and
asymptomatic children using reverse transcription-quantitative PCR. J
Med Virol. 2013;85:1661-8.
26. Ajjampur SS, Rajendran P, Ramani S, Banerjee I,
Monica B, Sankaran P, et al. Closing the diarrhoea diagnostic gap
in Indian children by the application of molecular techniques. J Med
Microbiol. 2008;57:1364-8.
27. Phan TG, Vo NP, Bonkoungou IJ, Kapoor A, Barro N,
O’Ryan M, et al. Acute diarrhea in West African children: diverse
enteric viruses and a novel parvovirus genus. J Virol. 2012;86:11024-30.
28. Ballal M, Shivananda PG. Rotavirus and enteric
pathogens in infantile diarrhoea in Manipal, South India. Indian J
Pediatr. 2002;69:393-6.
29. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D,
Farag TH, Panchalingam S, et al. Burden and aetiology of
diarrhoeal disease in infants and young children in developing countries
(the Global Enteric Multicenter Study, GEMS): A prospective,
case-control study. Lancet. 2013;382:209-22.
30. Curtis V, Cairncross S. Effect of washing hands
with soap on diarrhoea risk in the community: A systematic review.
Lancet Infect Dis. 2003;3:275-81.
|
|
|
 |
|

